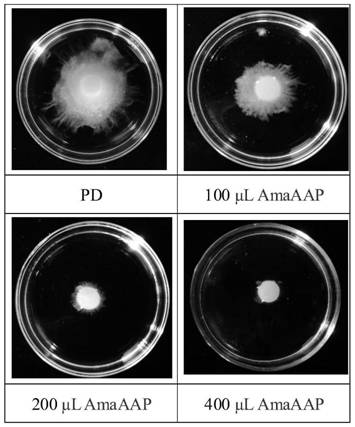
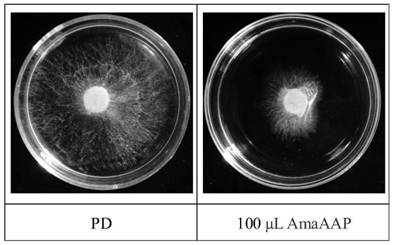
Amaranth recombinant antibacterial protein as well as preparation method and application thereof
A technology of recombinant protein and protein, which is applied in the direction of botanical equipment and methods, biochemical equipment and methods, applications, etc., to achieve the effects of less spots, obvious antibacterial effect, and reduced harm
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The present embodiment provides a kind of amaranth antibacterial protein AmaAAP:
[0037] The transmembrane region analysis, signal peptide analysis and hydrophobicity analysis of the amino acid sequence of amaranth antimicrobial protein Uniprot ID: Q96322 (gene name AAP, Gene ID: AAB67746.1) shows that the gene encodes 284 amino acids, and 6-26aa is the transmembrane region. In the membrane region, 1-31aa is the signal peptide sequence, the local hydrophilicity is good, and the expressed protein is expected to be soluble, and 32-284aa is selected for expression in Pichia pastoris. The amino acid sequence at positions 32-284 is the amino acid sequence shown in SEQ ID NO: 1, named as amaranth antibacterial protein AmaAAP.
Embodiment 2
[0039] This embodiment provides a gene encoding the above-mentioned amaranth antibacterial protein AmaAAP:
[0040] The amino acid sequence shown in SEQ ID NO: 1 was codon-optimized according to the codon preference of Pichia pastoris, and the optimized nucleotide sequence was SEQ ID No: 2, which was named the amaranth antibacterial protein AmaAAP gene.
Embodiment 3
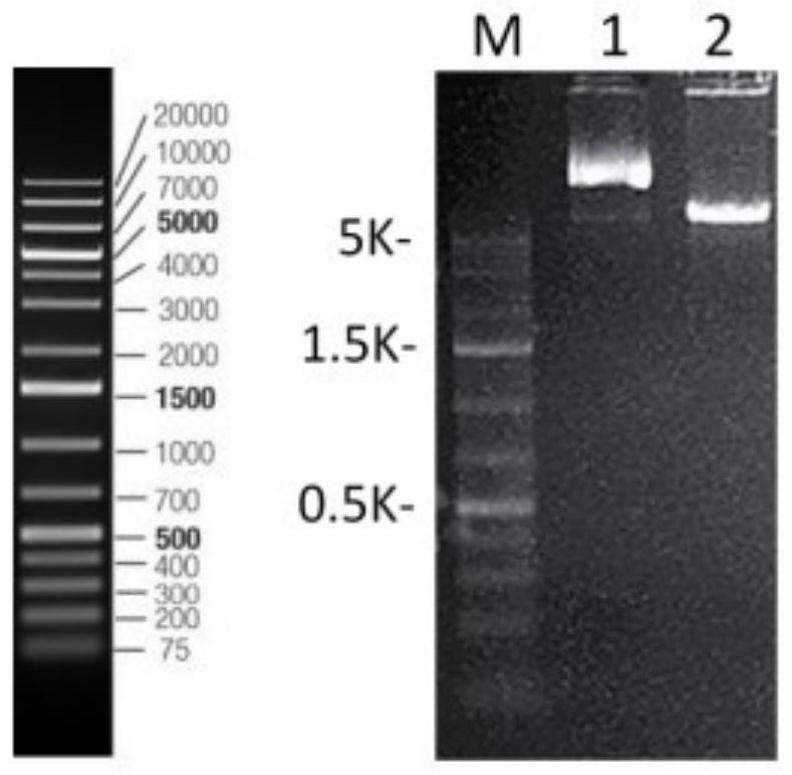
[0042] The present embodiment provides a kind of expression vector containing amaranth protein AmaAAP gene, and described expression vector is constructed through the following steps:
[0043] The nucleotide sequence obtained in Example 2 is as shown in SEQ ID No: 2. After the two ends of the amaranth antimicrobial protein AmaAAP gene are added with restriction site EcoRI / NotI and His tag, it is carried out to the whole gene synthesis to obtain the gene Fragment; the whole gene synthesis of the present embodiment is entrusted to Sangong Bioengineering (Shanghai) Co., Ltd. (hereinafter referred to as Sangong) to complete;
[0044] Use EcoRI / NotI to double digest the vector pPIC9K and the gene fragment obtained in the above steps, recover and purify, connect the double digested gene fragment to the vector pPIC9K, obtain the ligated product and transform it into Escherichia coli XL10 competent, pick The positive clones were amplified by PCR with the general primers of the pPIC9K ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com