Primers, method and test kit for detecting SNP site of gene PEAR1
A technology of PEAR1-CX-R and PEAR1-CX-F, which is applied in the fields of life science and biology, can solve the problems of high requirements for reaction conditions, cumbersome test process, and complicated operation process, so as to achieve simple and flexible operation and avoid false positives problem, reproducible effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] A kit for detecting the SNP site rs12566888 of the PEAR1 gene in a subject using HRM technology, including:
[0047] The sample DNA extraction reagent can be configured by yourself, or a ready-made kit can be purchased, such as the sample DNA extraction kit from QIAGEN.
[0048] HRM PCR reaction solution,
[0049] Standards and Negative Controls,
[0050] The HRM PCR reaction solution includes a pair of primers (PEAR1-HRM-F and PEAR1-HRM-R) for detecting the rs12566888 site, 2×HRM Analysis PreMix (Tiangen Biochemical Technology Co., Ltd.) and ddH 2 O, the primers for detecting the rs12566888 site are:
[0051] PEAR1-HRM-F:CACAGCTTGTAAGACAGAGATAGG;
[0052] PEAR1-HRM-R:AACCCTAGTTCCTTGCTCGG;
[0053] The standard product is GG genotype, GT genotype and TT genotype DNA; negative control substance: normal saline or without any substance.
[0054] In order to verify the results of HRM technology detection samples, the direct sequencing method is used for sequencing. Dur...
Embodiment 2
[0062] (1) Use the blood / cell genomic DNA extraction kit (QIAGEN) to extract whole blood genomic DNA. The operation process is as follows:
[0063] 1) Add 20μl QIAGEN Protease (or proteinase K) to the bottom of a 1.5ml centrifuge tube.
[0064] 2) Add 200 μL plasma to the centrifuge tube.
[0065] 3) Add 200 μl Buffer AL and shake for 15 seconds. (Note: Do not add QIAGEN Protease or proteinase K directly to Buffer AL. If the sample size is large, increase QIAGEN Protease and Buffer AL proportionally.)
[0066] 4) Water bath at 56°C for 10 minutes, and then briefly centrifuge to remove the liquid on the inner edge of the centrifuge tube cover.
[0067] 5) Add 200 μl of ethanol (96%-100%), shake for 15 s, and centrifuge briefly to remove the liquid along the inner edge of the centrifuge tube cover.
[0068] 6) Carefully add the mixture obtained above (including the precipitate) into the QIAamp Mini spin column (do not wet the edge), put the spin column into a 2ml collection t...
Embodiment 3
[0113] Embodiment 3 clinical sample detection
[0114] Take 20 cases of clinical samples to be tested, extract DNA solution, prepare reagents and detect according to the method described in Example 2.
[0115] Add 1 μL of the DNA solution extracted from each clinical sample to the detection system PCR reaction solution. At the same time, make a copy of each standard product. Detect with a fluorescent PCR instrument, and the time is 60 minutes.
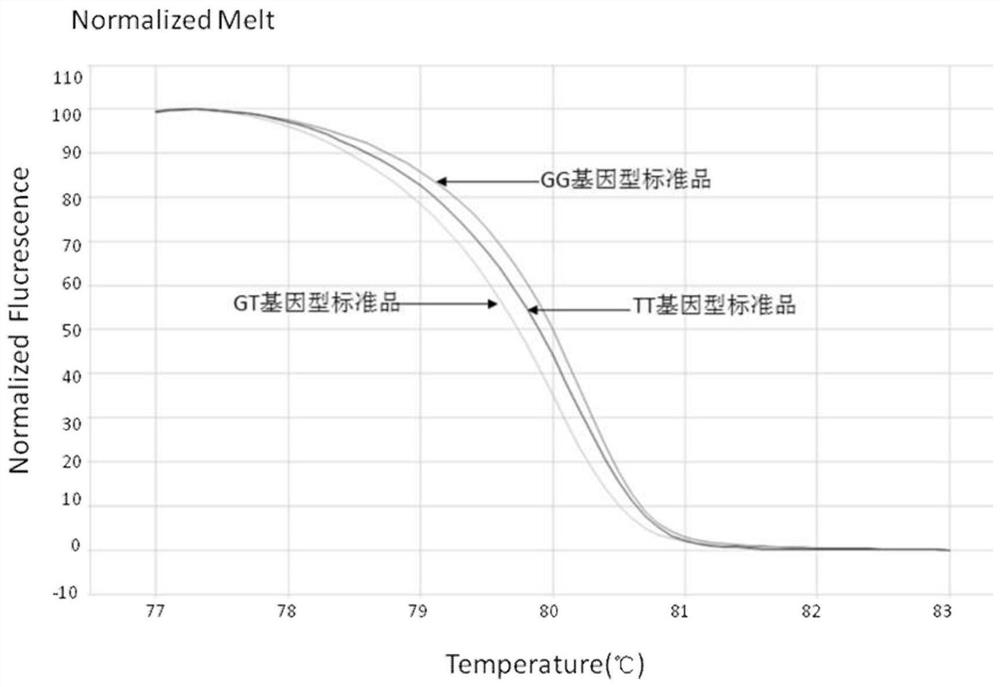
[0116] Such as figure 1 Shown are the Normalized Melting curves of standard TT, standard GT, and standard GG.
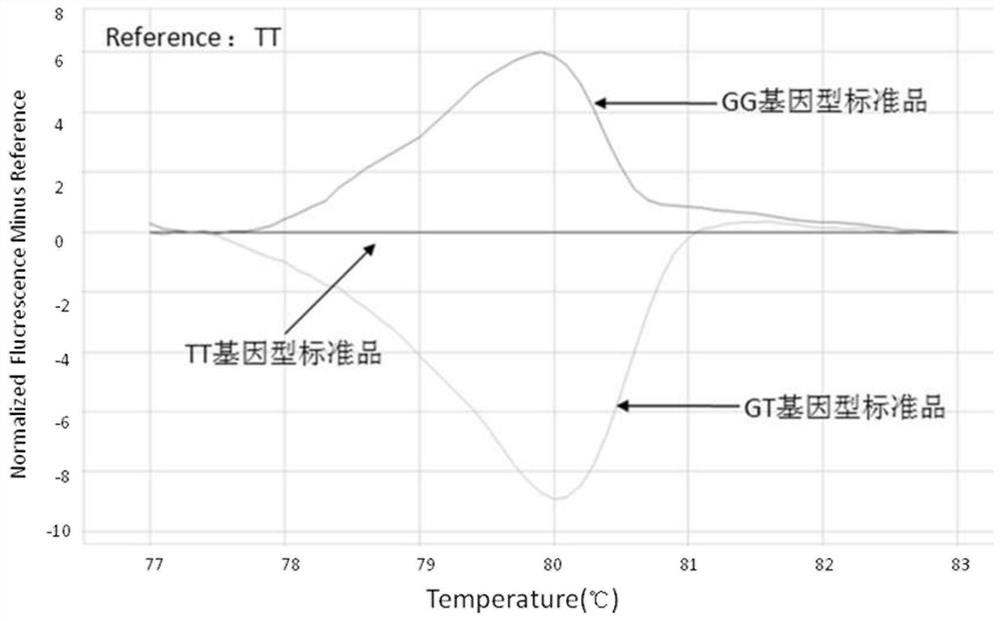
[0117] Such as figure 2 Shown are the Difference Melting curves for standard TT, standard GT, and standard GG.
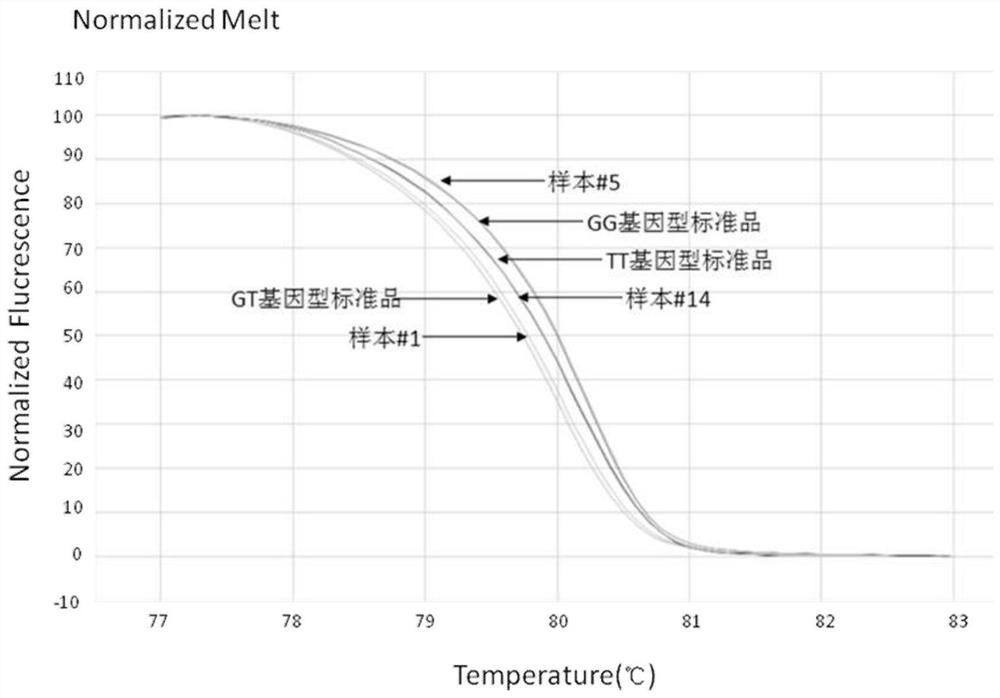
[0118] Such as image 3 Shown are the Normalized Melting curves for samples #1, #5, #14 and the standard.
[0119] Such as Figure 4 Shown are the Difference Melting curves for samples #1, #5, #14 and the standard.
[0120] According to the HRM results, the genotypes at the PEAR1 rs12566888 locus of 20 sc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com