Primer set for detecting duck hepatitis A virus type 3, kit and applications
A technology of duck hepatitis A virus and detection kit, which is applied in the direction of recombinant DNA technology, biochemical equipment and methods, and microbial measurement/inspection, can solve the problems of low sensitivity, difficulty in standardization, and long time consumption, and achieve high sensitivity Simple operation and fast effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
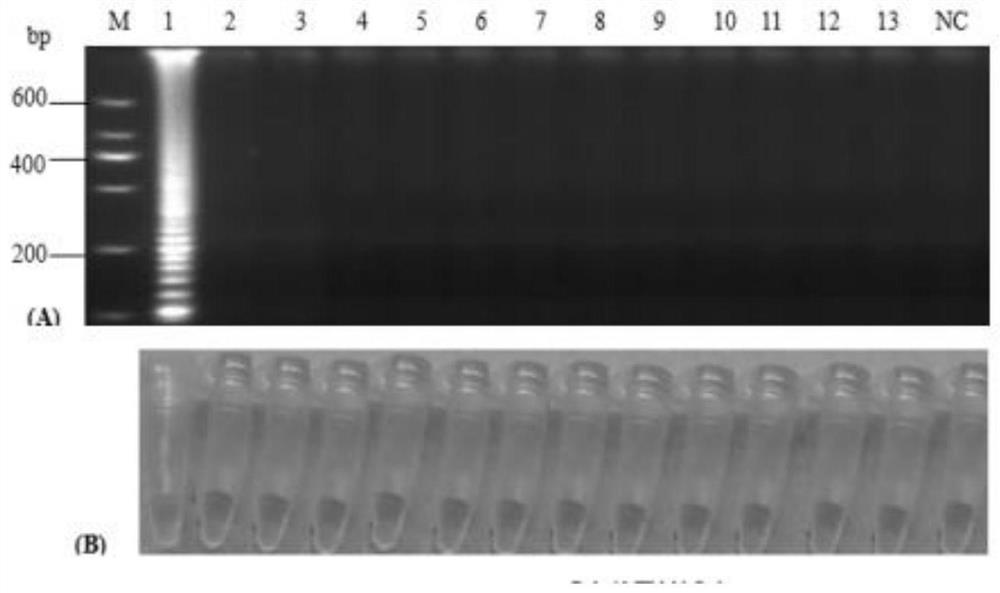
[0024] A primer set for detecting type 3 duck hepatitis A virus, comprising inner primers 1 and 2, outer primers 3 and 4, and the primer sequences are as follows:
[0025] Internal primer FIP: 5'-CACCACTGAATCTTGCCTTCA-ACACTCGTAAGATAGTTGGAG-3', SEQ ID No.1;
[0026] Internal primer BIP: 5'-CAAAGGCCCAGAAGCATTCAAA-GTAGTTGTAATATTCAGGACCAT-3', SEQ ID No.2;
[0027] Outer primer F3: 5'-AACACCTGCATTTCTGCC-3', SEQ ID No.3;
[0028] Outer primer B3: 5'-GAGCTTTCAACTTGGAACAA-3', SEQ ID No.4.
[0029] A kit containing the above primers, wherein the reaction solution: contains 10×BstBuffer, Bst DNA polymerase 8U / μL, 10mM dNTPs, 100mM magnesium sulfate, 20μM inner primer 1, 20μM inner primer 2, 20μM outer primer 3, 20μM outer primer 4 , 10M betaine, 625μM calcein, 12.5mM manganese chloride and double distilled water.
[0030] Specifically, the reaction solution is 24 μL per tube, and its composition is: 2.5 μL 10×BstBuffer, 1 μL Bst DNA polymerase 8U / μL, 3.5 μL 10 mM dNTPs, 2 μL 100 mM m...
Embodiment 2
[0032] The method for detecting type 3 duck hepatitis A virus by using the type 3 duck hepatitis A virus LAMP detection kit in Example 1 of the present invention.
[0033] 1. Extraction of RNA in tissue samples (total RNA extraction commercial kit)
[0034] A. Add 250 μL virus allantoic fluid and 750 μL lysate RZ to the centrifuge tube, shake and mix well, add 200 μL chloroform, shake vigorously for 30 seconds and place at room temperature for 3 minutes.
[0035] B. Centrifuge at 12000r / min at 4°C for 10min. The sample is divided into three layers: yellow organic layer, middle layer and supernatant colorless water phase. The extract mainly exists in the water phase. Transfer the water to a new centrifuge tube (about 600μL ).
[0036] C. Slowly add 0.5 times the volume (about 300 μL) of absolute ethanol, mix well and transfer the mixture into the adsorption column CR3. Centrifuge at 12000r / min at 4°C for 1min. (If the entire solution and mixture cannot be added to the adsorp...
Embodiment 3
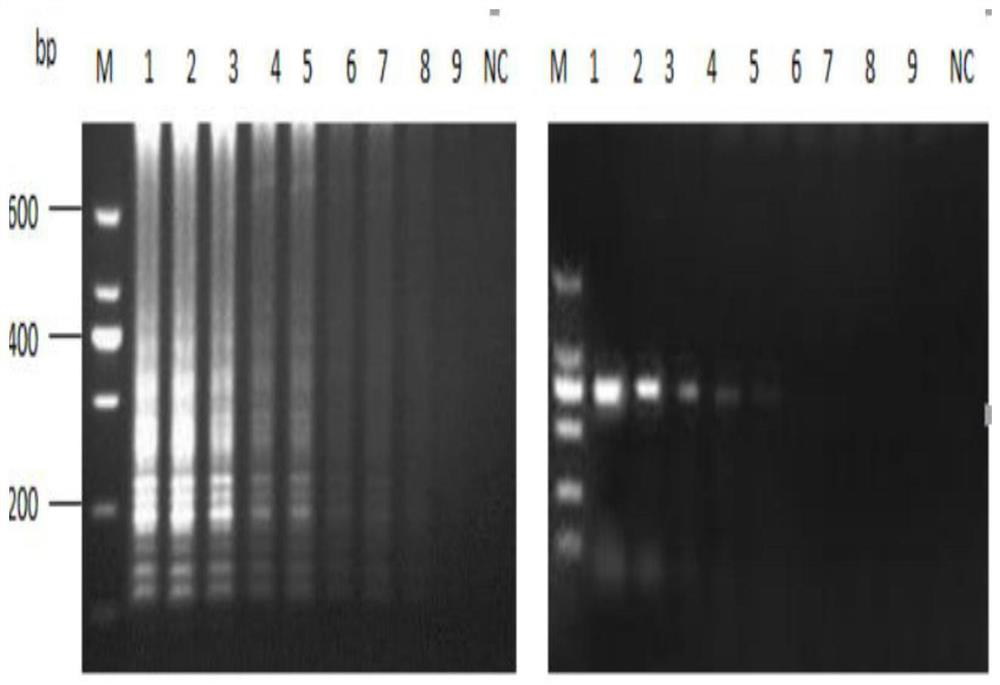
[0047] specificity test
[0048] DHAV-3 and DHAV-1, duck Tambusu virus (DTMUV), Newcastle disease virus (NDV), avian influenza virus (AIV), avian reovirus (ARV), duck plague virus (DEV), large intestine Bacillus, Pasteurella, Salmonella, Riemerella anatipestifer, Listeria, and Staphylococcus aureus were used as templates, and nucleic acids extracted from healthy poultry tissues and reverse-transcribed were used as negative controls. Viral LAMP should be reacted according to the system and conditions.
[0049] see results figure 1 , the detection of duck hepatitis A virus type 3 was positive (shown in green), while DHAV-1, DTMUV, NDV, AIV, ARV, DEV, Escherichia coli, Pasteurella, Salmonella, Riemerella anatipestifer, Listeria The virus tests of Tetrabacterium and Staphylococcus aureus were all negative (shown in orange). As a result of electrophoresis, only DHAV-3 had positive bands.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com