Preparation method and application of solid zinc oxide microspheres
A zinc oxide, solid technology, applied in the field of preparation of solid zinc oxide microspheres, can solve the problems of limited nano-zinc oxide photocatalysts, uneven particle size distribution, difficult to control parameters, etc., to improve the photocatalytic efficiency, uniform distribution, rough surface effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Take 3.1647g of cetyltrimethylammonium bromide and dissolve it in 40ml of absolute ethanol, ultrasonically dissolve for 20 minutes, then add 2.2001g of zinc acetate dihydrate, ultrasonically dissolve for 30 minutes, add 0.6033g of urea, ultrasonically dissolve for 50 minutes, and finally Transfer the mixed solution into the inner lining of the polytetrafluoroethylene reactor, raise the oven from room temperature to 140°C at 2 min / °C, and react for 4 hours. After the oven was cooled to room temperature, the sample was taken out for suction filtration, washed with absolute ethanol and water, and dried at 60°C for 10 hours to obtain solid zinc oxide microspheres.
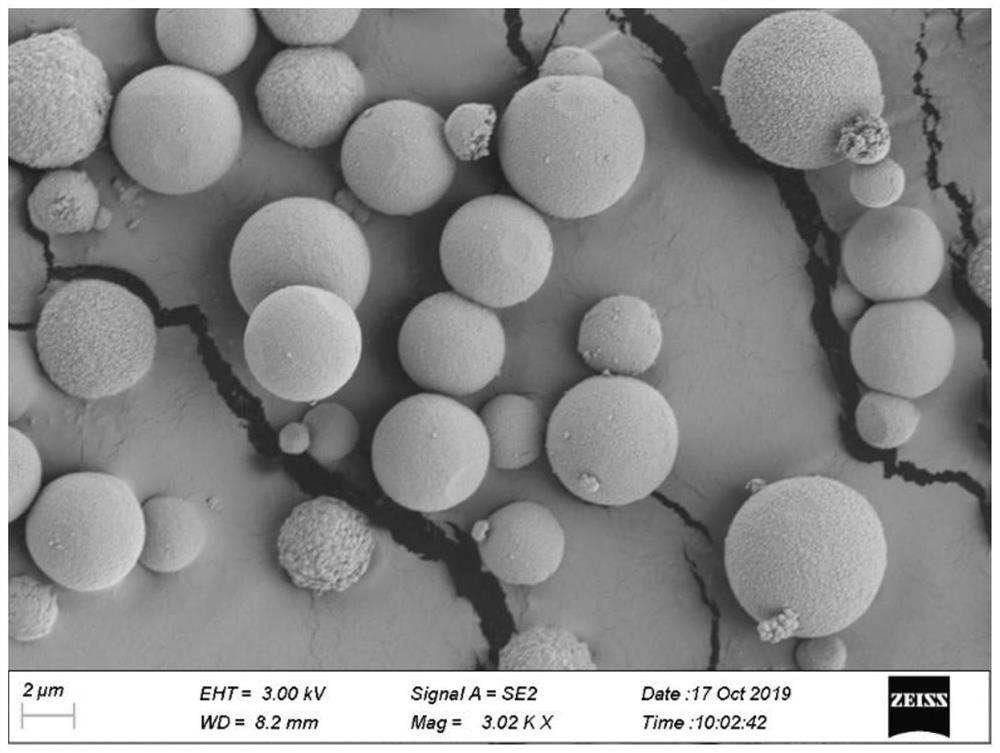
[0029] figure 1 For the preparation of solid zinc oxide microsphere scanning electron micrograph in this example, figure 2 For its partial enlarged view, from figure 2 It can be seen that the surface of solid zinc oxide microspheres is rough, the particle size is micron, and the specific surface area is larg...
Embodiment 2
[0034] Take 2.1868g of cetyltrimethylammonium bromide and dissolve it in 40ml of absolute ethanol, ultrasonically dissolve for 20 minutes, then add 2.1946g of zinc acetate dihydrate for ultrasonic dissolution for 30 minutes, then add 0.6009g of urea for ultrasonic dissolution for 30 minutes, and finally put The mixed solution was transferred into the lining of a polytetrafluoroethylene reactor, and the oven was raised from room temperature to 140°C at 2 min / °C for 6 hours to react. After the oven was cooled to room temperature, the sample was taken out for suction filtration, washed with absolute ethanol and water, and dried at 60°C for 10 hours to obtain solid zinc oxide microspheres.
Embodiment 3
[0036] Take 2.9146g of cetyltrimethylammonium bromide and dissolve it in 40ml of absolute ethanol, ultrasonically dissolve for 20 minutes, then add 2.2001g of zinc acetate, ultrasonically dissolve for 30 minutes, weigh and add 0.6033g of urea, ultrasonically dissolve for 50 minutes, and finally Transfer the mixed solution into the lining of a polytetrafluoroethylene reactor, and raise the oven from room temperature to 140°C at 2 min / °C for 8 hours to react. After the oven was cooled to room temperature, the sample was taken out for suction filtration, washed with absolute ethanol and water, and dried at 60°C for 10 hours to obtain solid zinc oxide microspheres.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com