Monocarbazole/dicarbazole hole transport material as well as preparation method and application thereof
A hole transport material and biscarbazole technology, applied in the field of single/bicarbazole hole transport materials and their preparation, can solve the problems of lack of hole transport materials, complex synthesis routes, harsh reaction conditions, etc., and achieve high application Value, low cost, effect of improving molecular packing and hole transport capability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Synthesis of Example 1 Hole Transport Material A1
[0069] 1. Synthesis of compound 1
[0070]
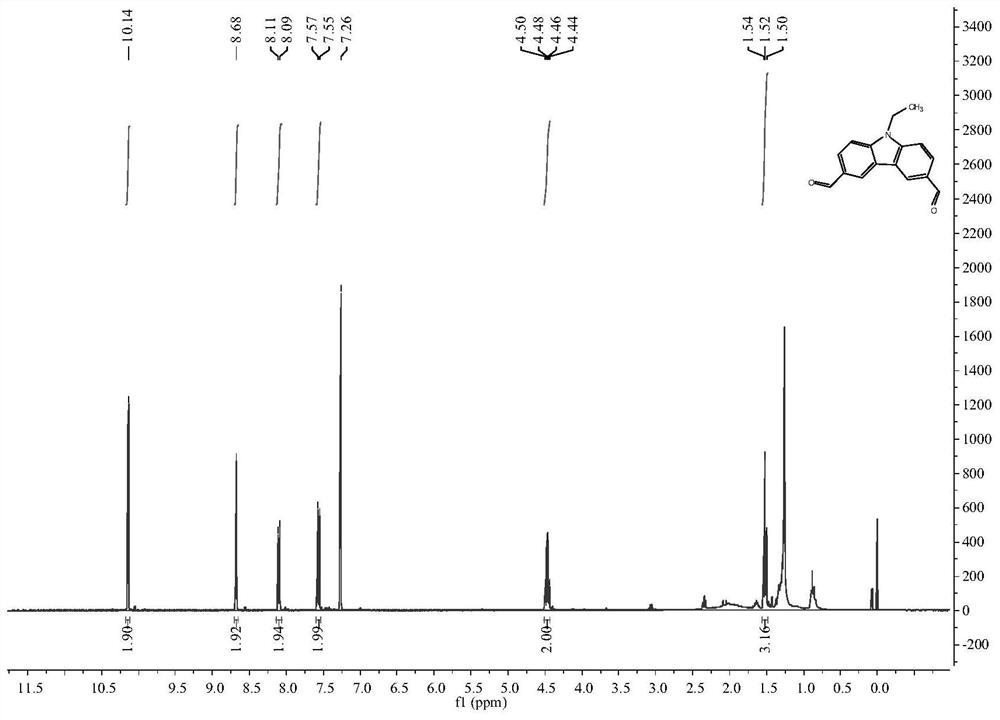
[0071] The reaction device was treated with anhydrous and protected by argon. Add DMF (12.3mL, 160.00mmol, 20.00eq) into a 25mL two-necked flask, stir in an ice-water bath for 20min, then slowly add POCl 3 (7.5mL, 80.00mmol, 10.00eq), with dried CH 2 Cl 2 Dissolve N-ethylcarbazole (1.5621g, 8.00mmol, 1.00eq) in (6.0mL), slowly drop into the two-necked flask at room temperature, stir for 10min, heat at 90°C for reaction, stop the reaction after 21h, and saturated saline / acetic acid Ethyl extraction was performed, the organic layer was dried over anhydrous magnesium sulfate, and column chromatography (ethyl acetate: petroleum ether = 1:3, v / v) yielded 1.2402 g of the target product with a yield of 61.7%. 1 H NMR (400MHz, CDCl 3 ): δ(TMS,ppm)=10.14(s,2H),8.68(s,2H),8.10(d,J=8Hz,2H),7.56(d,J=8Hz,2H),4.47(q,J =8Hz, 2H), 1.52(t, J=8Hz, 3H), such as figure 1 As shown, com...
Embodiment 2
[0084] Synthesis of Example 2 Hole Transport Material A4
[0085] 1. Synthesis of Compound 5
[0086]
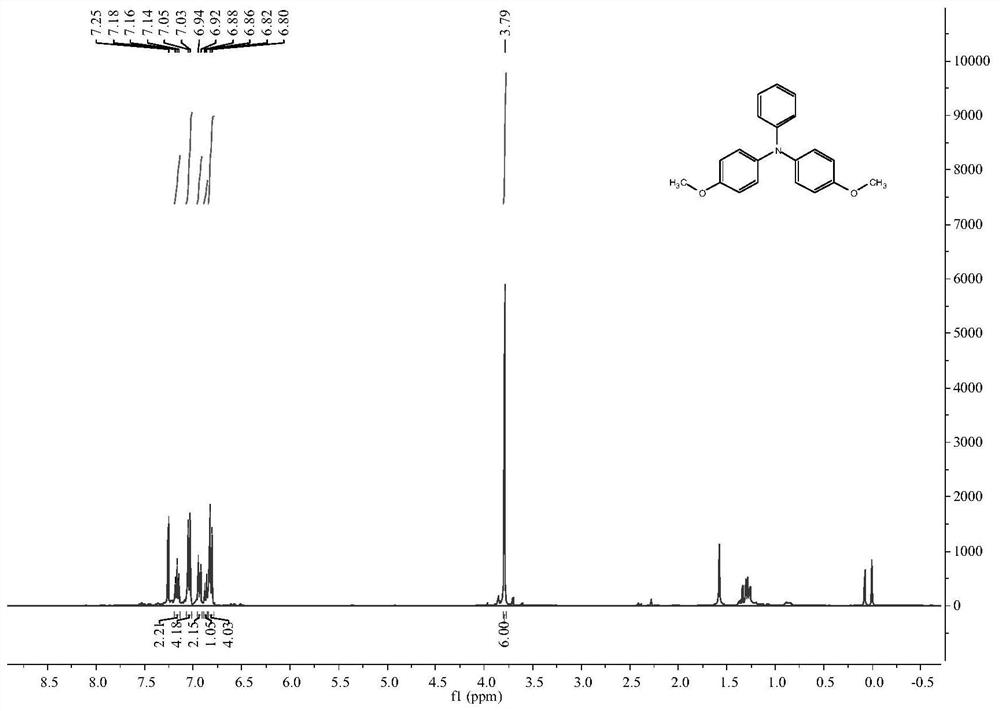
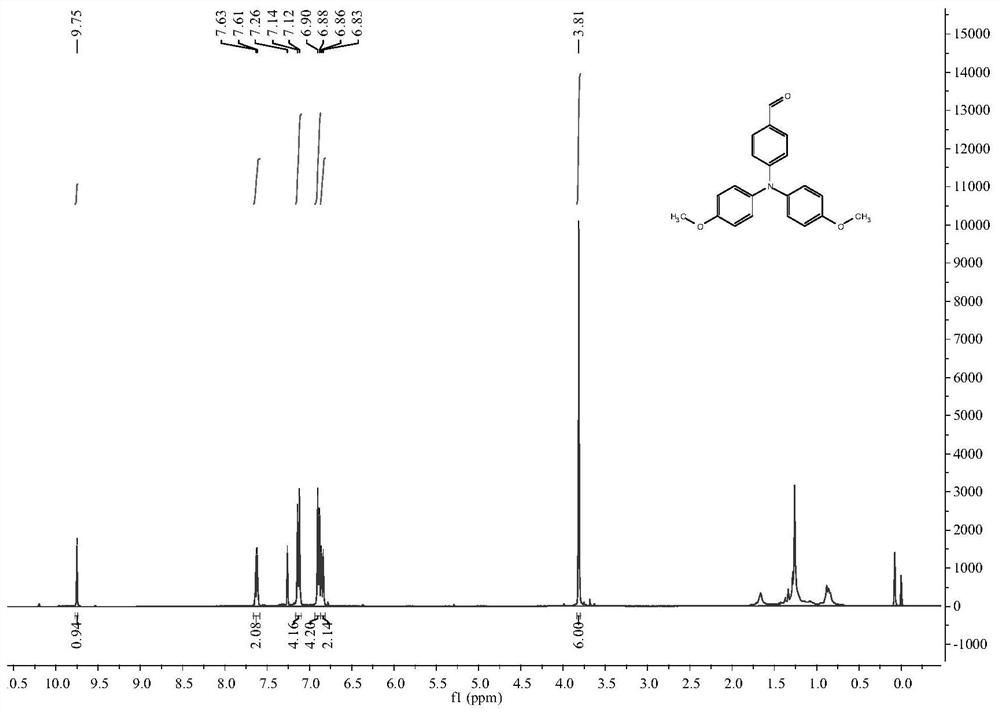
[0087] The reaction device was treated with anhydrous and protected by argon. Add compound 2 (0.1527g, 0.50mmol, 1.00eq.), N-iodosuccinimide (0.1237g, 0.55mmol, 1.10eq.) and N,N-dimethylformamide into a 25mL two-necked flask Amide (4.0mL), react at room temperature for 23h, extract with saturated brine / ethyl acetate, dry the organic layer with anhydrous magnesium sulfate, and perform column chromatography (ethyl acetate:petroleum ether=1:50, v / v) to obtain the target product 0.2100 g, yield 97.4%. 1 H NMR (400MHz, CDCl 3 ): δ(TMS,ppm)=7.30(d,J=8Hz,2H),6.93(d,J=8Hz,4H),6.72(d,J=8Hz,4H),6.58(d,J=8Hz, 2H),3.68(s,6H), such as Image 6 As shown, compound 5 was obtained.
[0088] 2. Synthesis of Compound 6
[0089]
[0090] The reaction device was treated with anhydrous and protected by argon. Add compound 5 (0.1941g, 0.45mmol, 1.00eq.), trimethylsilylacetylene (0.08...
Embodiment 3
[0097] Example 3 Optical and electrical performance characterization of hole transport materials A1 and A4 and their application in the preparation of perovskite solar cells
[0098] The above-mentioned synthesized hole-transport materials A1 and A4 and the existing hole-transport material spiro-OMeTAD were tested for photophysical properties (ultraviolet, fluorescence), electrochemical properties, etc. The specific test methods and results are as follows:
[0099] (1) Ultraviolet-visible absorption spectrum: the instrument model is Shimadzu UV-3600, and the sample is scanned at a wavelength of 200-800nm, and the results are as follows Figure 10 As shown, it shows that A1 and A4 mainly absorb around 300-400nm, effectively avoiding the absorption in the visible light region, and have little influence on the light absorption of the perovskite layer. The spectral onset absorption wavelength can be obtained by the formula △E g =hc / λ onset , to calculate the band gap width.
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com