Aryl naproxen derivative high-valence iodine compound, preparation method and application thereof
A technology for aryl naproxen and derivatives, which is applied in the preparation of organic compounds, chemical instruments and methods, preparation of sulfonates, etc., can solve the problems of narrow application range, poor selectivity, lack of naproxen derivatives, etc. , to achieve the effect of concise and clear structure modification, high reactivity and easy preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] A kind of preparation of hypervalent iodine trifluoromethanesulfonate of phenylnaproxen methyl ester comprises the following steps:
[0041] 1) Add 10 mmol of hydroxy (p-toluenesulfonyloxy) iodobenzene to a mixed solution of 80 mL of dichloromethane and 8 mL of 2,2,2-trifluoroethanol, then add 10 mmol of naproxen methyl ester compound, and cool the reaction solution After reaching 0°C, 10 mmol of trimethylsilyl trifluoromethanesulfonate was added dropwise, and then reacted at room temperature for 1 h.
[0042] 2) After the reaction was completed, the solvent was distilled off under reduced pressure, and diethyl ether was added to stir and precipitate to obtain a white solid product with a yield of 88%.
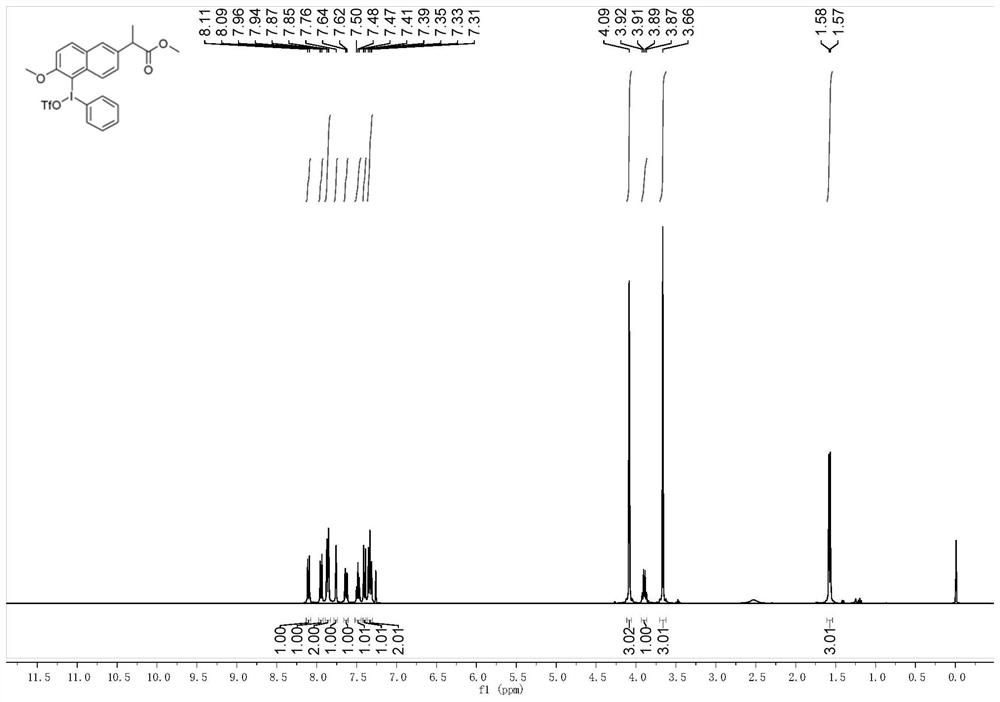
[0043] NMR characterization of hypervalent iodine trifluoromethanesulfonate of phenylnaproxen methyl ester as follows figure 1 Shown: 1H NMR (400MHz, CHLOROFORM-D) δ8.10 (d, J = 9.0Hz, 1H), 7.95 (d, J = 8.7Hz, 1H), 7.86 (d, J = 7.5Hz, 2H), 7.76(s,1H),7.63(d,J=7.1Hz,1H...
Embodiment 2
[0045]A kind of preparation of hypervalent iodine trifluoromethanesulfonate of 4-methylphenylnaproxen methyl ester comprises the following steps:
[0046] 1) In a 250mL round-bottomed flask, dissolve 10mmol of 4-methyliodobenzene in 100mL of dichloromethane, add 10mmol of m-chloroperoxybenzoic acid under stirring conditions, then add 10mmol of toluenesulfonic acid monohydrate, at room temperature Stir at low temperature for 30 minutes. After the reaction is complete, spin the solvent to dryness, add 150 mL of ether, and stir thoroughly for 30 minutes to form a precipitate. After filtration and drying in vacuum, the yield of hydroxy(p-toluenesulfonyloxy)4-methyliodobenzene was 98%.
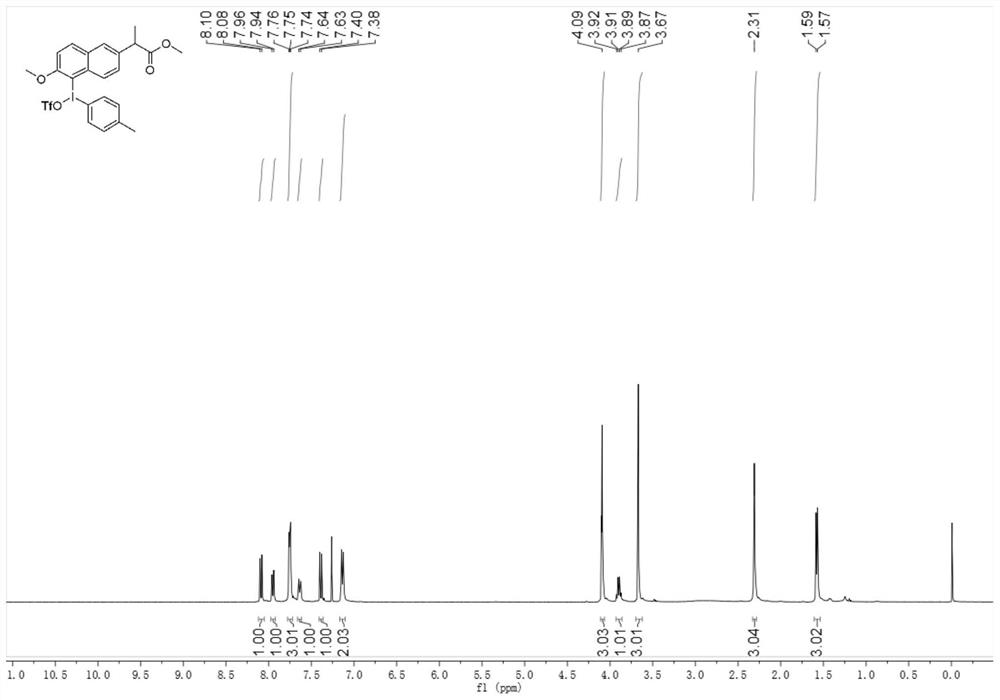
[0047] 2) In a 250mL round bottom flask, add 8mmol of the hydroxyl group (p-toluenesulfonyloxy) obtained in step 1) into a mixed solution of 65mL of dichloromethane and 6.5mL of 2,2,2-trifluoroethanol or hexafluoroisopropanol 8 mmol of naproxen methyl ester compound was then added, the reaction so...
Embodiment 3
[0051] A kind of preparation of hypervalent iodine trifluoromethanesulfonate of 2.4.6-trimethylphenylnaproxen methyl ester, comprises the following steps:
[0052] 1) In a 250mL round bottom flask, dissolve 10mmol of iodine in 80mL of dichloromethane, add 20mmol of mesitylene under stirring conditions, then add 30mmol of m-chloroperoxybenzoic acid and 20mmol of p-toluenesulfonic acid monohydrate, The reaction was carried out at room temperature for 1 hour. After the reaction was completed, the solvent was spin-dried, and 150 mL of diethyl ether was added, and stirred thoroughly for 30 minutes to form a precipitate. After filtration and drying in vacuo, the yield of hydroxy(p-toluenesulfonyloxy)-me-trimethyliodobenzene was 97%.
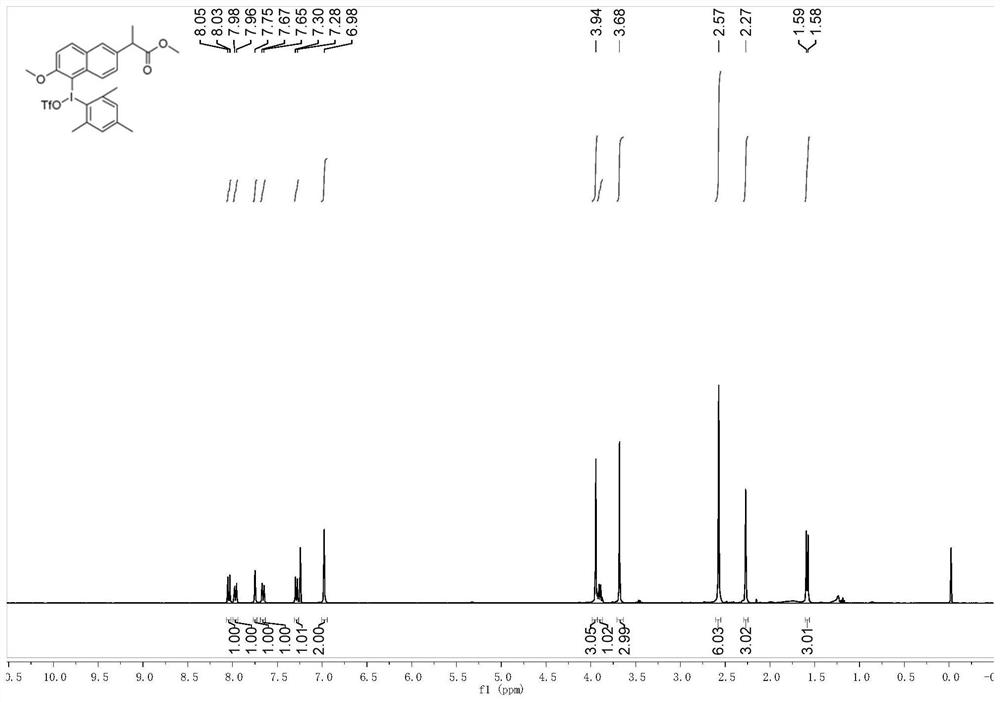
[0053] 2) In a 250mL round-bottomed flask, add 8mmol of hydroxy(p-toluenesulfonyloxy)s-trimethyliodobenzene obtained in step 1) into 65mL of dichloromethane and 2,2,2-trifluoroethanol or hexafluoroiso To a mixed solution of 6.5 mL of propanol, 8 mmol ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com