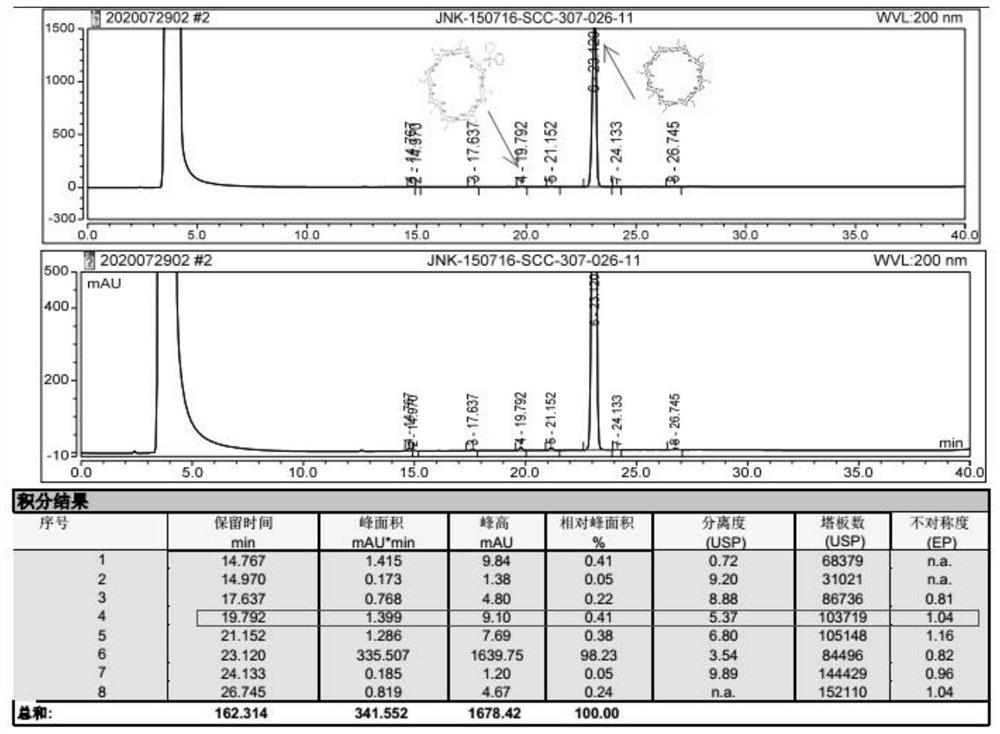
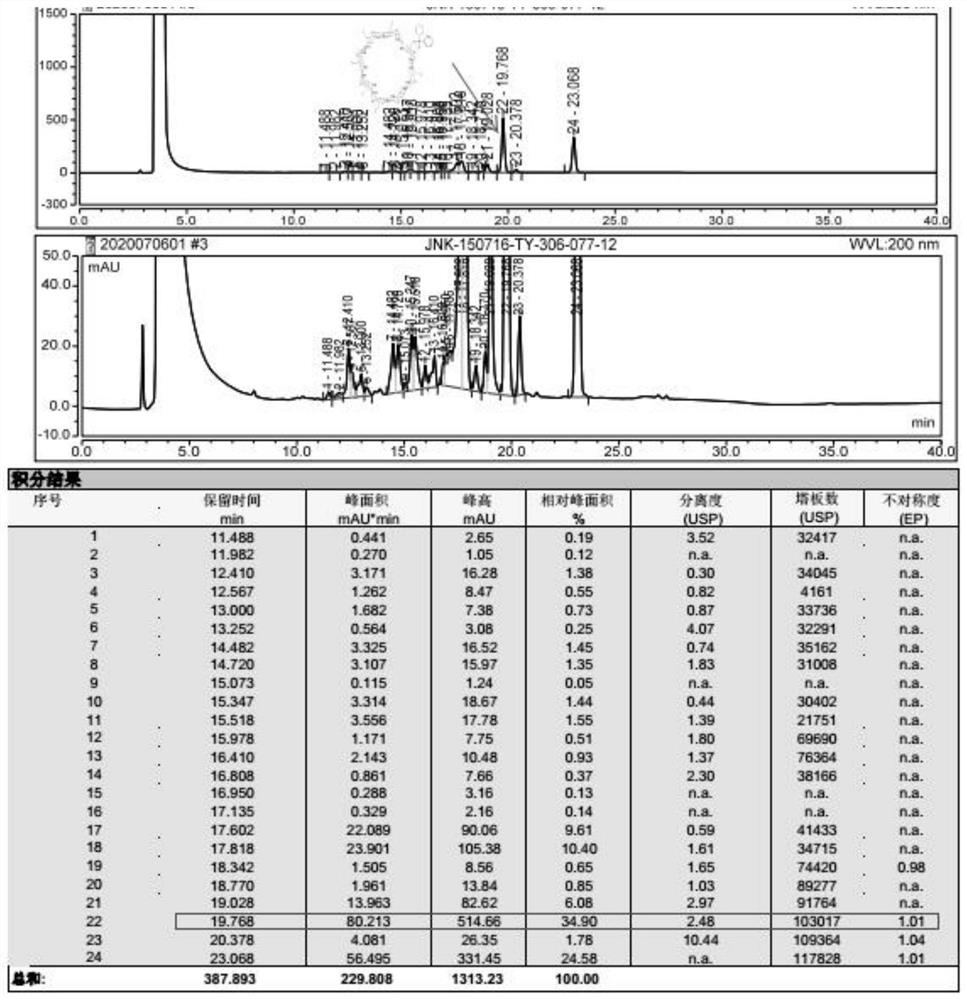
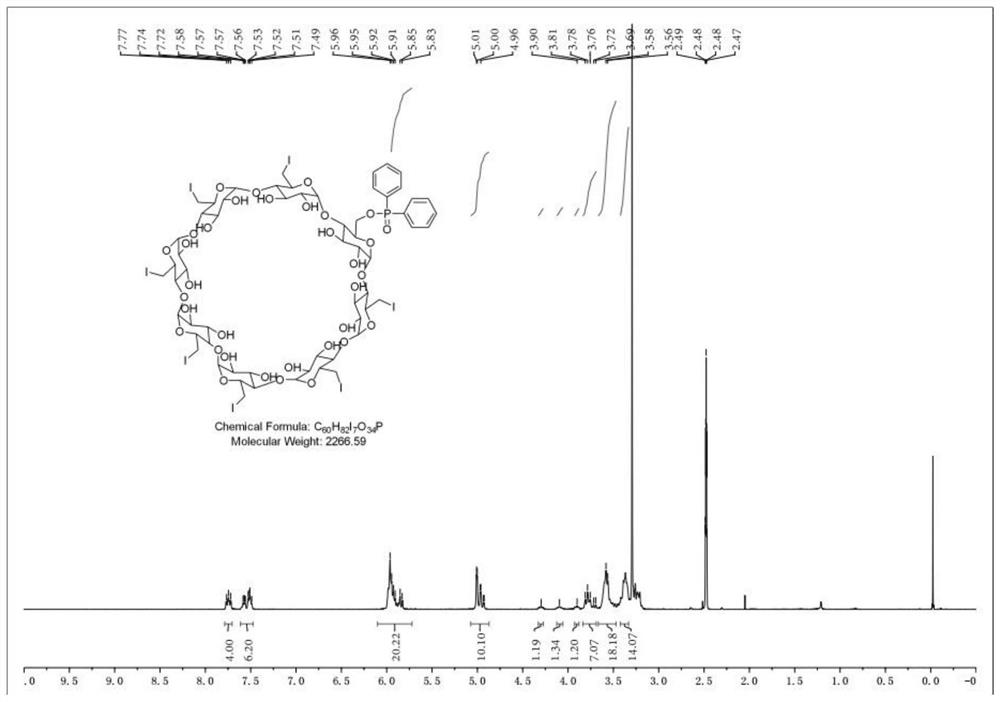
Sugammadex sodium intermediate diphenylphosphinic acid derivative impurity and preparation method thereof
A technology of sugammadex sodium and diphenyl phosphate, which is applied in the field of drug impurity synthesis, can solve the problem that the impurity of sugammadex sodium diphenyl phosphate has not been reported, and the research on impurities of diphenyl phosphate derivatives has not been mentioned, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1 A sugammadex sodium intermediate diphenyl phosphate derivative impurity, its preparation method is as follows:
[0038] Under nitrogen protection, add N,N-dimethylformamide (10.5ml) and diphenylphosphoric acid (2.87g, 13.16mmol) into a 100ml three-necked flask, control the internal temperature to ≤10°C, and then add cesium carbonate (4.29 g, 13.16mmol), stirring and reacting for 0.5 hours, while stirring and reacting, add N,N-dimethylformamide (18ml) in a 100ml beaker, then add 6-full deoxy-6-full iodo-γ-ring Dextrin (3.58g, 1.65mmol), stirred until the solids were completely dissolved and set aside, after the stirring reaction was completed for 0.5 hours, add the above-mentioned 6-perdeoxy-6-periodo-γ-cyclodextrin dropwise into a 100ml three-necked flask. Solution, after the dropwise addition, heat up to 60-65°C for 18 hours, then cool down to 20-30°C, add 100ml of purified water to the reaction system, stir at room temperature for 1h, filter, and filter c...
Embodiment 2
[0047] Embodiment 2 A sugammadex sodium intermediate diphenyl phosphate derivative impurity, its preparation method is as follows:
[0048]At room temperature, under the protection of nitrogen, add dimethylsulfoxide (5.3ml) and diphenylphosphoric acid (0.91g, 4.15mmol) into a 100ml three-necked flask, control the internal temperature to ≤10°C, and then add sodium hydroxide (0.17g, 4.15mmol), stirred and reacted for 0.5 hour, while stirring and reacted, dimethyl sulfoxide (9ml) was added in a 100ml beaker, and then 6-full deoxy-6-full iodo-γ-cyclodextrin (1.79g, 0.83mmol), stir until the solids are all dissolved and set aside, after the stirring reaction is completed for 0.5 hours, add the above-mentioned 6-perdeoxy-6-periodo-γ-cyclodextrin solution dropwise into a 100ml three-necked bottle, after the dropwise addition , heat up to 60-65°C and react for 18 hours, then cool down to 20-30°C, add 50ml of purified water to the reaction system, stir at room temperature for 1 hour, f...
Embodiment 3
[0049] Embodiment 3 A sugammadex sodium intermediate diphenyl phosphate derivative impurity, its preparation method is as follows:
[0050] At room temperature, under argon protection, add dimethyl sulfoxide (10.5ml) and diphenylphosphoric acid (4.31g, 19.74mmol) to a 100ml three-necked flask, control the internal temperature to ≤10°C, and then add 60% sodium hydride (0.79 g, 19.74mmol), stirring and reacting for 0.5 hour, while stirring and reacting, add dimethyl sulfoxide (18ml) in a 100ml beaker, then add 6-full deoxy-6-full iodo-γ-cyclodextrin (3.58 g, 1.65mmol), stir until the solids are all dissolved and set aside, after the stirring reaction is completed for 0.5 hours, add the solution of the above-mentioned 6-perdeoxy-6-periodo-γ-cyclodextrin dropwise to a 100ml three-necked bottle, and add dropwise After completion, raise the temperature to 60-65°C for 18 hours, then lower the temperature to 20-30°C, add 100ml of purified water to the reaction system, stir at room tem...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com