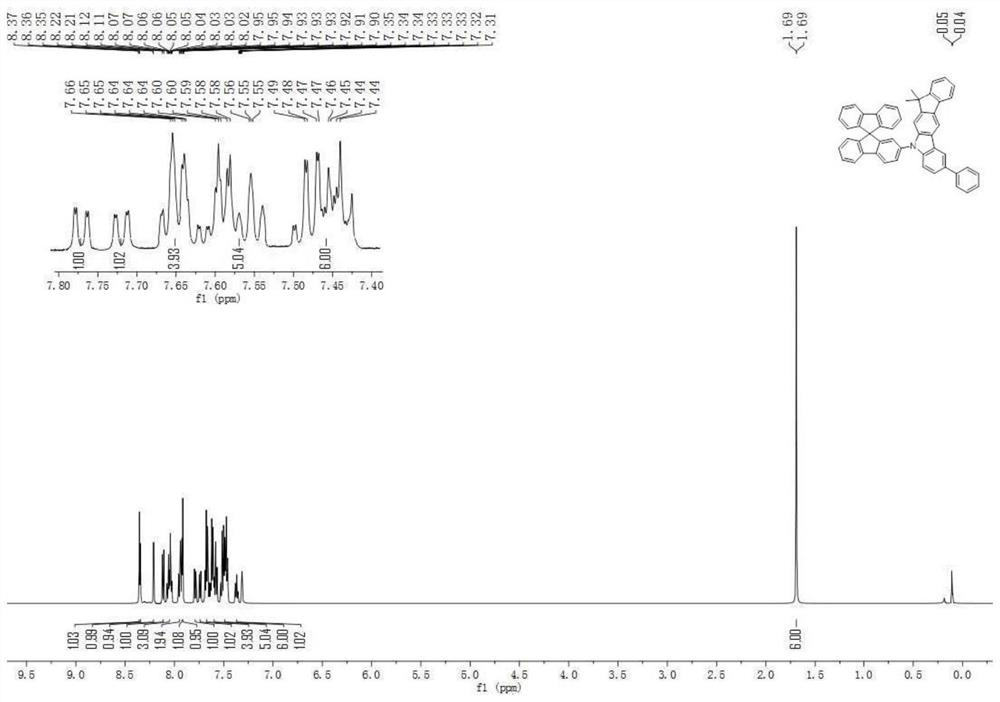
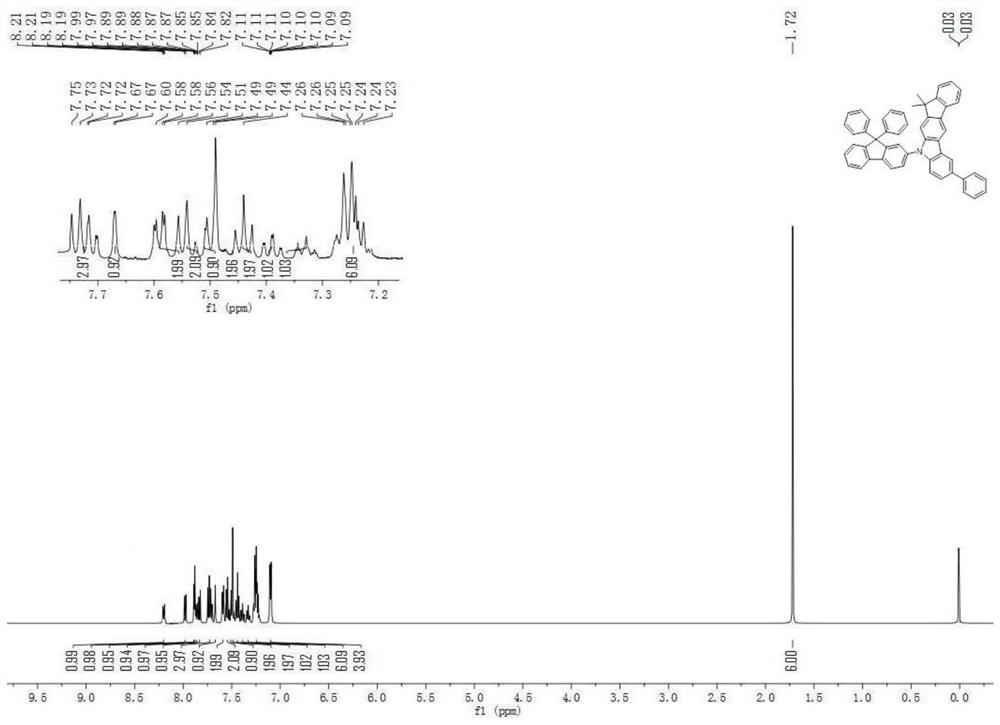
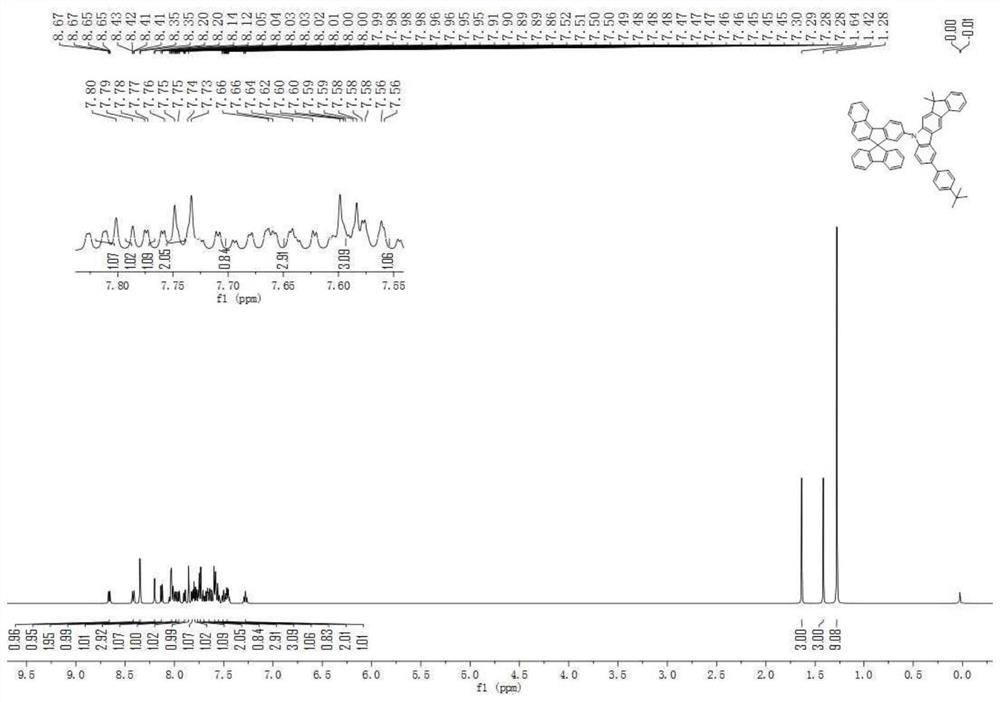
An organic electroluminescent device comprising fluorene derivatives
An electroluminescence device and luminescence technology, which is applied in the direction of electric solid-state devices, organic chemistry, electrical components, etc., can solve the problems of low glass transition temperature, poor device stability, and low triplet energy level, so as to improve the glass transition temperature , charge dispersion, life-enhancing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0095] The preparation method of the organic compound of the present invention can be prepared through the following synthetic route, but the present invention is not limited thereto:
[0096] 1, the synthesis of compound of the present invention
[0097]
[0098]2. Synthesis of intermediate S5 / S5'
[0099]
[0100] R 1 ~R 3 、Ar 1 ~ Ar 3 , L 1 , E, and a definition are the same as those defined above; Xa, Xb, Xc, and Xd are respectively any one of Br, Cl, and I.
[0101] Further, the organic layer further includes a hole injection layer, and the hole injection layer includes an axene compound represented by formula IV,
[0102]
[0103] Among them, R 2 , R 3 , R 4 Any one independently selected from substituted C6-C30 aryl groups and substituted C3-C30 heteroaryl groups;
[0104] The substituted group is selected from any one of fluorine, chlorine, bromine, iodine, cyano, trifluoromethyl or nitro.
[0105] Preferably, the axene compound represented by the f...
Embodiment 1
[0141] [Example 1] Synthesis of Compound 1
[0142]
[0143] Synthesis of Intermediate S3-1
[0144] Intermediate S2-1 (6.0g, 21mmol) was dissolved in 500mL of N,N-dimethylformamide (DMF), and N-bromosuccinyl was added to the mixture within 30min at room temperature in the dark. Imine (NBS) (3.7g, 21mmol), the resulting mixture was stirred for 8 hours, after the reaction was completed, the reactant was poured into Na 2 CO 3 solution (3M, 1L), the resulting product was then washed with dichloromethane, the organic phase was separated and the solvent was removed using a rotary evaporator. Subsequently, the solid was recrystallized to obtain intermediate S3-1 (7.0 g, 92%), and the purity of the solid was ≥99.8% as determined by HPLC.
[0145] Mass Spectrum m / z: 361.0645 (Theoretical: 361.0466). Theoretical element content (%)C 21 h 16 BrN: C, 69.63; H, 4.45; Br, 22.06; N, 3.87. Measured element content (%): C, 69.60; H, 4.47; Br, 22.03; N, 3.90. The above results confi...
Embodiment 2
[0152] [Example 2] Synthesis of Compound 7
[0153]
[0154] Synthesis of Intermediate S4-2
[0155] The phenylboronic acid in Example 1 was replaced by an equimolar amount of phenyl-D5-boronic acid, and the intermediate S4-2 (4.4g, 68%) was prepared according to the synthesis method of Example 1, and the solid purity was detected by HPLC≥99.1 %.
[0156] Mass Spectrum m / z: 364.2133 (Theoretical: 364.1988). Theoretical element content (%)C 27 h 16 D. 5 N: C, 88.97; H, 7.19; N, 3.84. Measured element content (%): C, 88.94; H, 7.20; N, 3.86. The above results confirmed that the obtained product was the target product.
[0157] Synthesis of compound 7
[0158] Intermediate S4-1 in Example 1 was replaced by an equimolar amount of Intermediate S4-2, and compound 7 (5.1 g, 75%) was prepared according to the synthesis method of Example 1, and the solid purity was ≥99.4% as determined by HPLC.
[0159] Mass Spectrum m / z: 678.3235 (Theoretical: 678.3083). Theoretical eleme...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com