Novel hyaluronic acid-hydrolyzing enzyme mutant and pharmaceutical composition comprising same
A composition and variant technology, applied in the field of novel human hyaluronidase variants, can solve problems such as insufficient thermal stability or expression level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0186] Example 1. Construction of PH20 variants
[0187]To construct PH20 variants, the cDNA of wild-type PH20 (clone ID: hMU002604) was purchased from Korean Human Gene Bank. Wild-type PH20 encodes amino acids from L36 to S490. The PH20 gene was amplified by polymerase chain reaction (hereinafter referred to as PCR), and inserted into the XhoI and NotI restriction enzyme sites of the pcDNA3.4-TOPO vector. For expression in ExpiCHO cells, the signal peptide of human growth hormone, human serum hormone, or human Hyal1 was used as the signal peptide instead of the original signal peptide of PH20. For protein purification using HisTrap columns, the DNA sequence of the 6xHis tag is located at the 3' end of the PH20 cDNA. Amino acid substitutions of the PH20 variants were made using the PCR method and confirmed by DNA sequencing.
[0188] The list of primers used to clone PH20 variants is summarized in Table 9 below and the specific sequences of the primers are summarized in Tab...
Embodiment 2
[0227] Example 2. Construction of PH20 variants HM1 and HM6
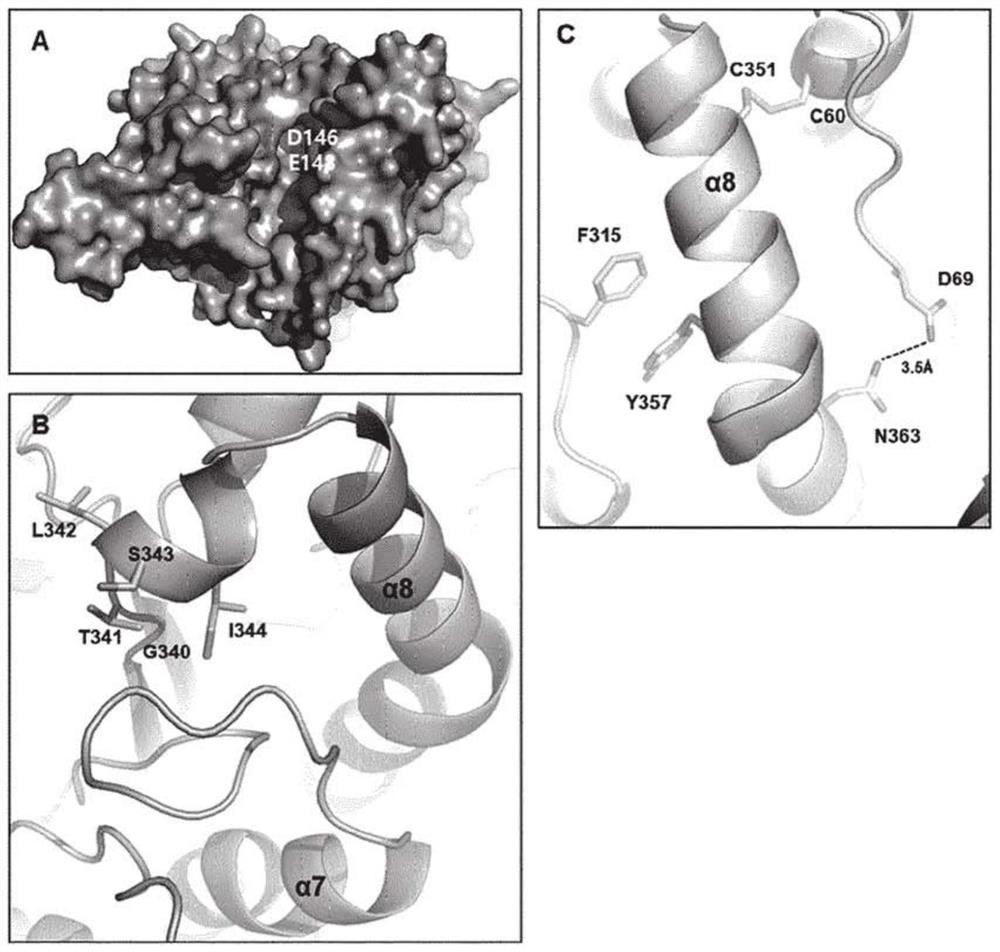
[0228] Such as figure 1 As shown in B, amino acid residues M345 to N363 of PH20 correspond to the α-helix 8 region and the linker region between α-helix 7 and α-helix 8 in the protein tertiary structure model. Among the amino acids of α-helix 8, C351 forms a disulfide bond with C60 of α-helix 1; Y357 forms a hydrophobic interaction with F315 of α-helix 7; and N363 forms a hydrogen bond with D69 of α-helix 1, thereby making the The secondary structure is stable ( figure 1 C).
[0229] To construct variants with higher enzymatic activity and thermostability than WT by substituting amino acids located in the α-helix 8 region of PH20 and the linker region between α-helix 7 and α-helix 8, the following variants were constructed: variant HM1, in which 12 amino acids in the region M345 to N363 were substituted; variant HM2, in which 7 amino acids in the region Y365 to V379 were substituted; and variant HM3, in which 19 ...
Embodiment 3
[0233] Example 3. Construction of PH20 variants HM4, HM7, HM8, HM9, HM10, HM11 and HM12
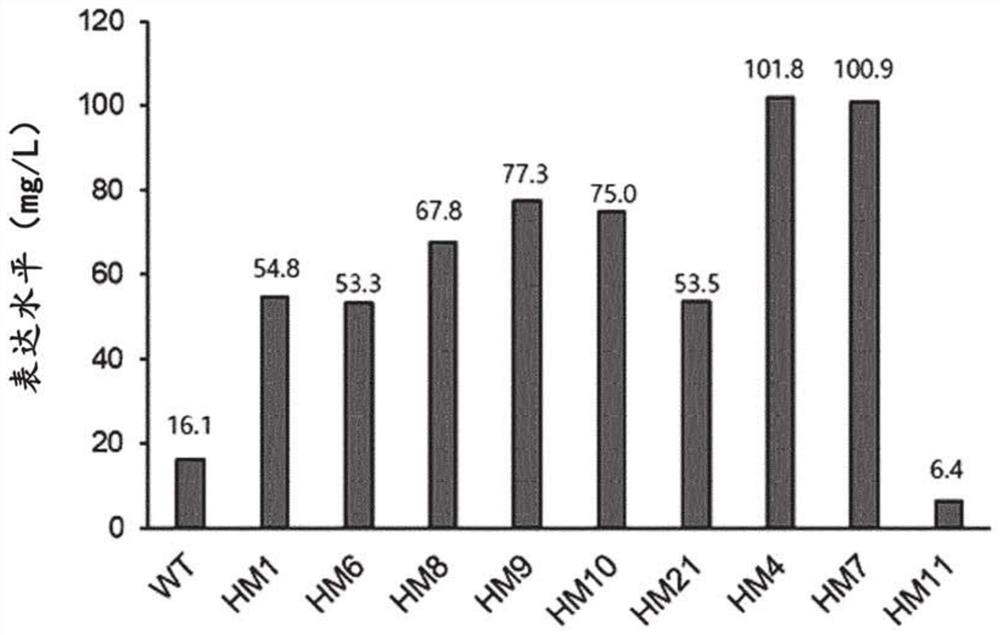
[0234] It is believed that through the construction of variants HM1 and HM6 in Example 2, the amino acid substitutions in the M345 to N363 and M345 to I361 regions resulted in an increase in both enzymatic activity and thermostability of PH20, a great advance in protein engineering. Therefore, based on the variants HM1 and HM6, other amino acids in the N-terminal and C-terminal direction were additionally substituted.
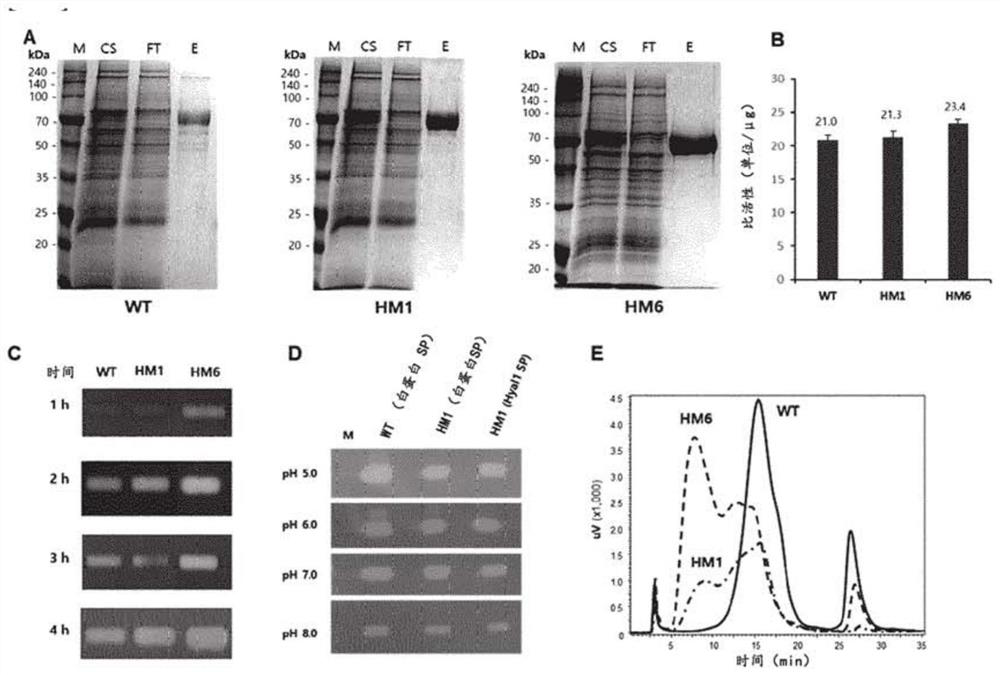
[0235] First, variants HM4 and HM7 were constructed, comprising the substituted amino acids in variants HM1 and HM6, and wherein the amino acids between G340 and I344 were additionally substituted by G340V, T341S, L342W, S343E and I344N . The sequences of the substituted amino acid sequences in variants HM4 and HM7 are shown in Table 11 above. Variants HM4 and HM7 were expressed in ExpiCHO cells. The protein purification results of HM7 are in Figure 5 Shown in A. HM4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com