Preparation method of 3-sulfuryl quinoline compound
A technology of sulfone quinoline and compounds, which is applied in the field of preparation of 3-sulfone quinoline compounds, can solve the problems that are not suitable for large-scale industrial application and will cause problems, and achieve strong substrate designability and simple preparation process , strong practical effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
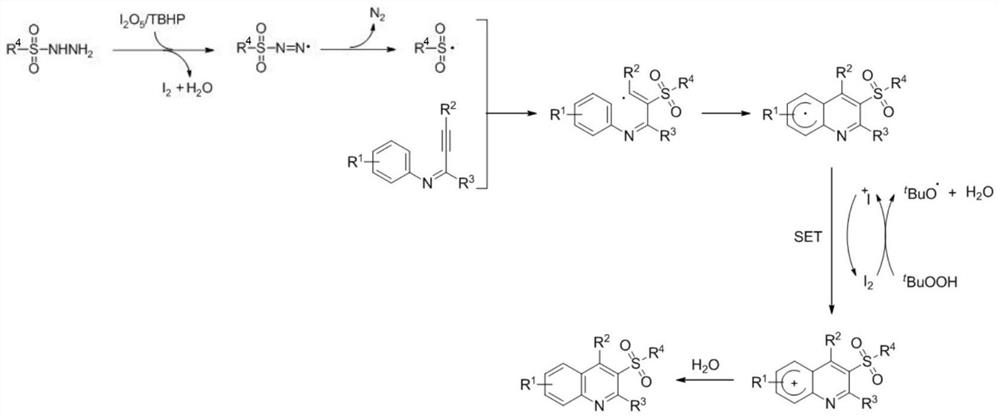
[0035] A kind of preparation method of 3-sulfonyl quinoline compound, with diiodine pentoxide as initiator, peroxy tert-butanol as oxidant, by mixing alkynyl imine compound and sulfonyl hydrazide compound, cyclization reaction occurs, 3-sulfonyl quinoline compounds were obtained.
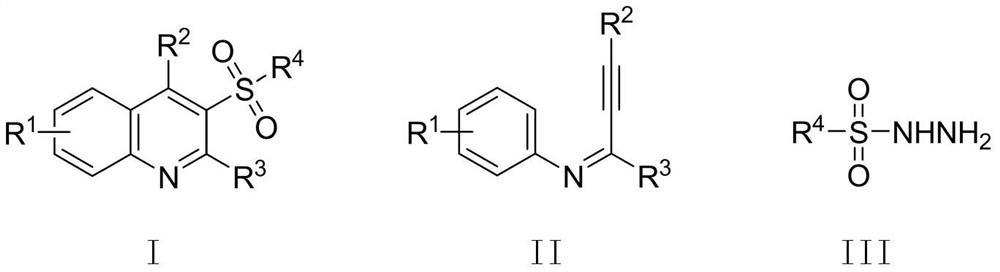
[0036] The structure of the 3-sulfone quinoline compound is shown in formula I; the structure of the alkynyl imine compound is shown in formula II: the structure of the sulfonyl hydrazide compound is shown in formula III:
[0037]
[0038] In the formula:
[0039] R 1 One selected from hydrogen, p-chloro or p-methyl;
[0040] R 2 One selected from phenyl, thienyl or cyclopropyl;
[0041] R 3 One selected from phenyl, tert-butyl or hydrogen;
[0042] R 4 One selected from phenyl or thienyl.
[0043] Described preparation method comprises the steps:
[0044] (1) At a certain temperature, add an alkynyl imine compound, a sulfonyl hydrazide compound and an organic solvent into the test tube ...
Embodiment 2
[0057] Take a 25mL test tube reactor, add 0.5mmol alkynyl imine, 0.6mmol sulfonyl hydrazide and 5mL acetonitrile. At 70° C., 0.5 mmol of diiodine pentoxide and 2.5 mmol of tert-butanol peroxide were added to the above reaction system, and stirred for 8 hours. The progress of the reaction was detected by TLC (petroleum ether: ethyl acetate = 3:1). After the reaction is complete, add 20 mL of saturated ammonium chloride aqueous solution to quench the reaction, separate the organic phase, extract the aqueous phase three times with ether (50 mL each time), combine the organic phases, dry over anhydrous sodium sulfate, remove the solvent, and use petroleum ether: ethyl acetate Esters=3:1 eluent column chromatography, to obtain a white solid, which is 3-sulfone quinoline compounds, yield: 74%, its structural formula is as follows:
[0058]
[0059] Its NMR ( 1 HNMR and 13 C NMR) detection data are:
[0060] 1 H NMR (500MHz, CDCl 3 )δ8.19(d,J=8.0Hz,1H),8.05–7.94(m,5H),7.76–7...
Embodiment 3
[0063] Take a 25mL test tube reactor, add 0.5mmol alkynyl imine, 0.6mmol sulfonyl hydrazide and 5mL acetonitrile. At 70° C., 0.5 mmol of diiodine pentoxide and 2.5 mmol of tert-butanol peroxide were added to the above reaction system, and stirred for 8 hours. The progress of the reaction was detected by TLC (petroleum ether: ethyl acetate = 3:1). After the reaction is complete, add 20 mL of saturated ammonium chloride aqueous solution to quench the reaction, separate the organic phase, extract the aqueous phase three times with ether (50 mL each time), combine the organic phases, dry over anhydrous sodium sulfate, remove the solvent, and use petroleum ether: ethyl acetate Esters=3:1 eluent column chromatography, to obtain a white solid, which is 3-sulfone quinoline compounds, yield: 71%, its structural formula is as follows:
[0064]
[0065] Its NMR ( 1 HNMR and 13 C NMR) detection data are:
[0066] 1 H NMR (500MHz, CDCl 3 )δ8.09(d,J=9.0Hz,1H),7.95–7.84(m,5H),7.76–7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com