Synthesis method of alpha-ketoamide compound
A synthesis method and technology for ester compounds, which are applied in the fields of amide synthesis and organic synthesis, can solve the problems of complicated reaction operation, harsh reaction conditions and high production cost, achieve simple synthesis steps, high product yield and purity, and avoid post-production problems. processing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Add 0.5 mmol of phenyl isocyanate compound and 0.6 mmol of benzoylformic acid to a 25 ml screw-top test tube successively, stir and react at 60°C for 6 hours, and cool to room temperature after the reaction to obtain the crude product , the crude product was purified by column chromatography to obtain the product 3aa with a yield of 92% and a purity of 99%.
[0063] The synthetic route is:
[0064]
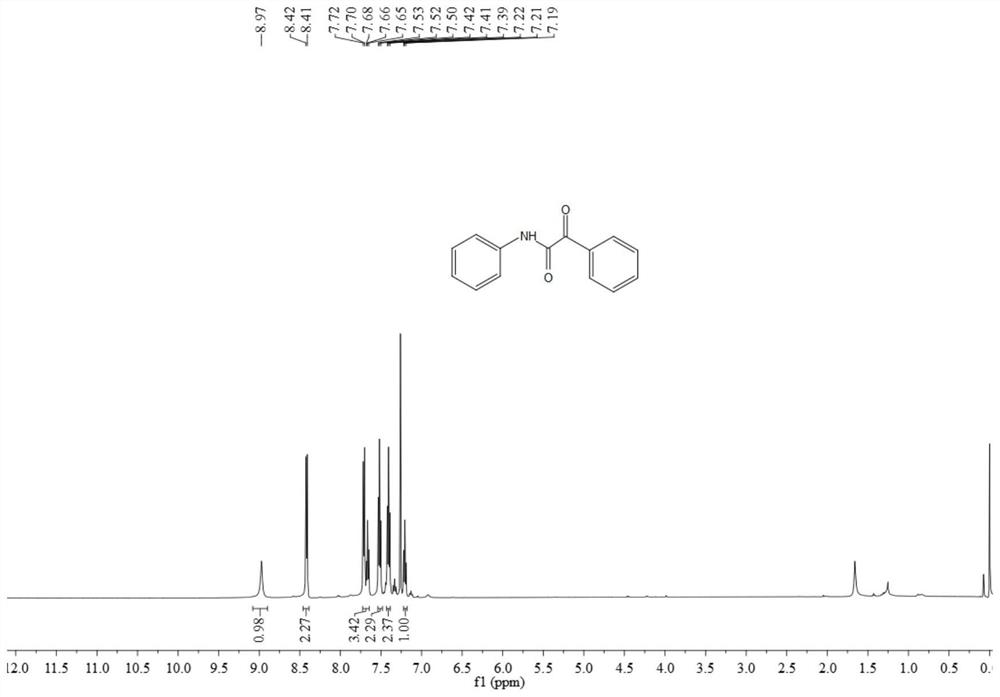
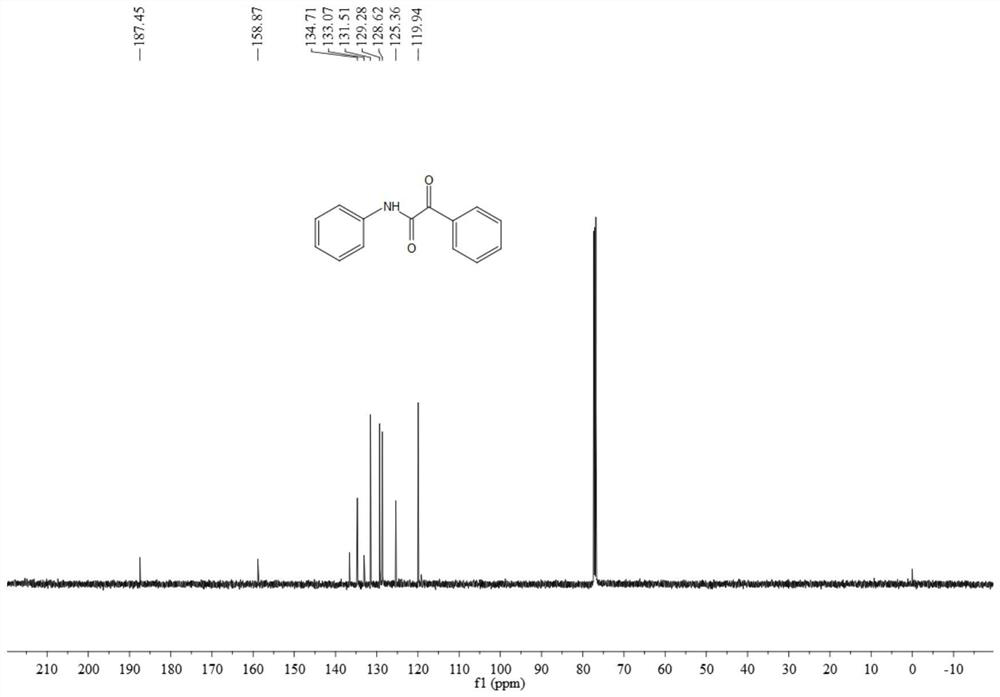
[0065] The resulting product 3aa is a pale yellow solid, and its hydrogen spectrum and carbon spectrum are as follows figure 1 with figure 2 As shown, the structural characterization data are as follows:
[0066] 1 H NMR (500MHz, CDCl 3 )δ8.97(s,1H),8.42(d,J=7.6Hz,2H),7.73-7.64(m,3H),7.52(t,J=7.7Hz,2H),7.41(t,J=7.9 Hz,2H),7.21(t,J=7.4Hz,1H);
[0067] 13 C NMR (126MHz, CDCl 3 ) δ 187.5, 158.9, 136.6, 134.7, 133.1, 131.5, 129.3, 128.6, 125.4, 119.9.
Embodiment 2
[0069] Add 0.5 mmol of 4-chlorophenylisocyanate and 0.6 mmol of benzoylformic acid to a 25 ml screw-top test tube in turn, stir and react at 60°C for 6 hours, and cool to room temperature after the reaction to obtain The crude product was purified by column chromatography to obtain product 3ad with a yield of 86% and a purity of 99%.
[0070] The synthetic route is:
[0071]
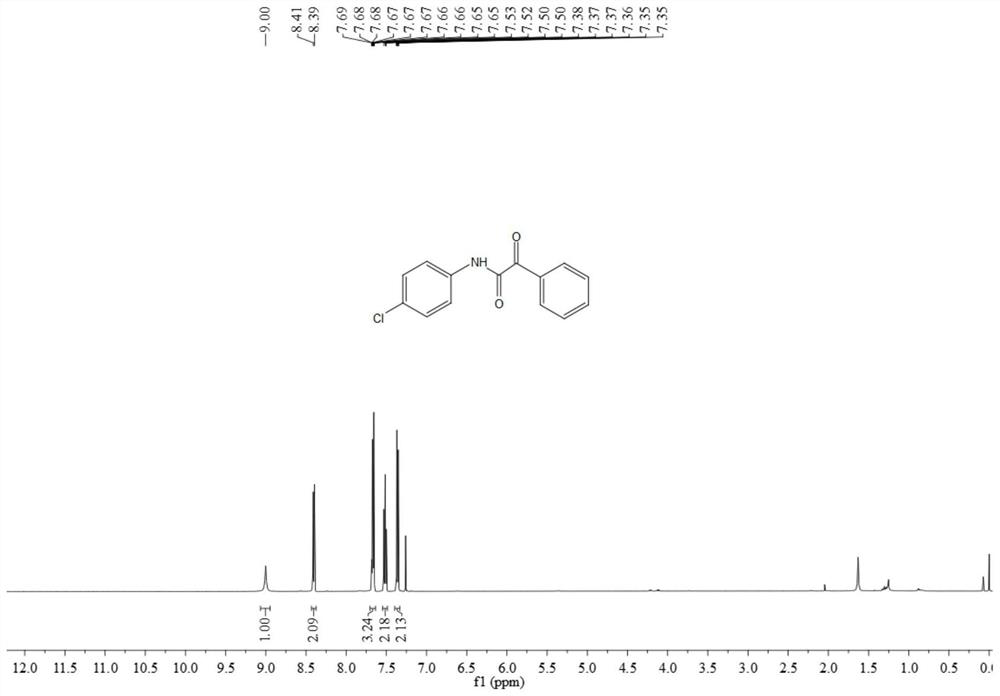
[0072] The resulting product 3ab is a light yellow solid, and its hydrogen spectrum and carbon spectrum are as follows image 3 with Figure 4 As shown, the structural characterization data are as follows:
[0073] 1 H NMR (500MHz, CDCl 3 )δ9.00(s,1H),8.40(dd,J=8.3,1.1Hz,2H),7.70-7.63(m,3H),7.51(dd,J=10.9,4.8Hz,2H),7.40-7.33 (m,2H);
[0074] 13 C NMR (126MHz, CDCl 3 ) δ 187.1, 158.8, 135.2, 134.8, 132.9, 131.5, 130.4, 129.3, 128.7.
Embodiment 3
[0076] Add 0.5 mmol of 4-iodophenylisocyanate and 0.6 mmol of benzoylformic acid to a 25 ml screw-top test tube in turn, stir and react at 60°C for 8 hours, and cool to room temperature after the reaction to obtain The crude product was purified by column chromatography to obtain the product 3ac with a yield of 79% and a purity of 98%.
[0077] The synthetic route is:
[0078]
[0079] The resulting product 3ac is a light yellow solid, and its hydrogen spectrum and carbon spectrum are as follows Figure 5 with Image 6 As shown, the structural characterization data are as follows:
[0080] 1 H NMR (500MHz, CDCl 3 )δ8.99 (s, 1H), 8.39 (dt, J = 8.5, 1.4Hz, 2H), 7.72-7.63 (m, 3H), 7.49 (ddt, J = 9.7, 4.8, 2.2Hz, 4H);
[0081] 13 C NMR (126MHz, CDCl 3 ) δ 187.0, 158.9, 138.2, 136.4, 134.8, 132.9, 131.5, 128.6, 121.7, 88.9.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com