Nitrogen-doped iron nanotube and preparation method and application thereof
A technology of nitrogen doping and iron nanometers, applied in chemical instruments and methods, catalyst activation/preparation, water/sludge/sewage treatment, etc., can solve poor nitrate removal performance, low nitrogen selectivity, acid-base tolerance Poor performance and other problems, to achieve the effect of high NO3- reduction performance, high conductivity, high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
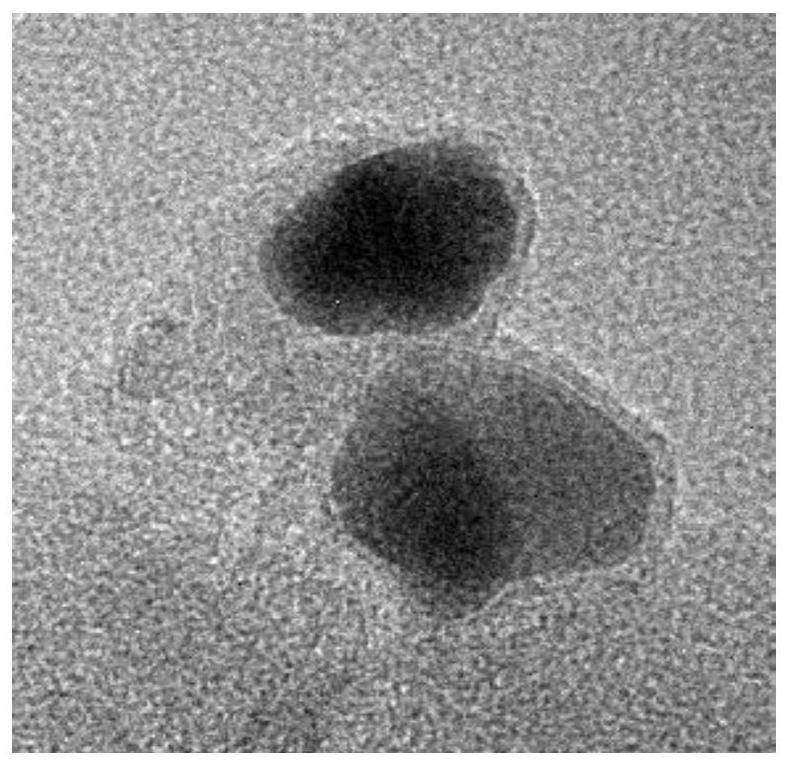
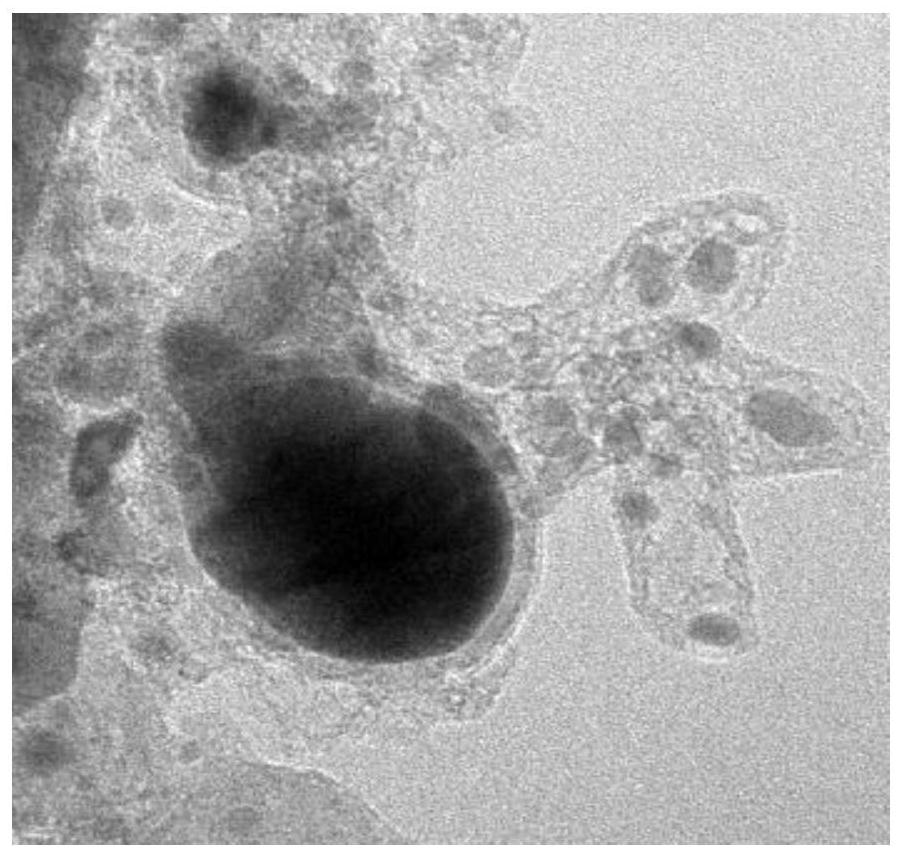
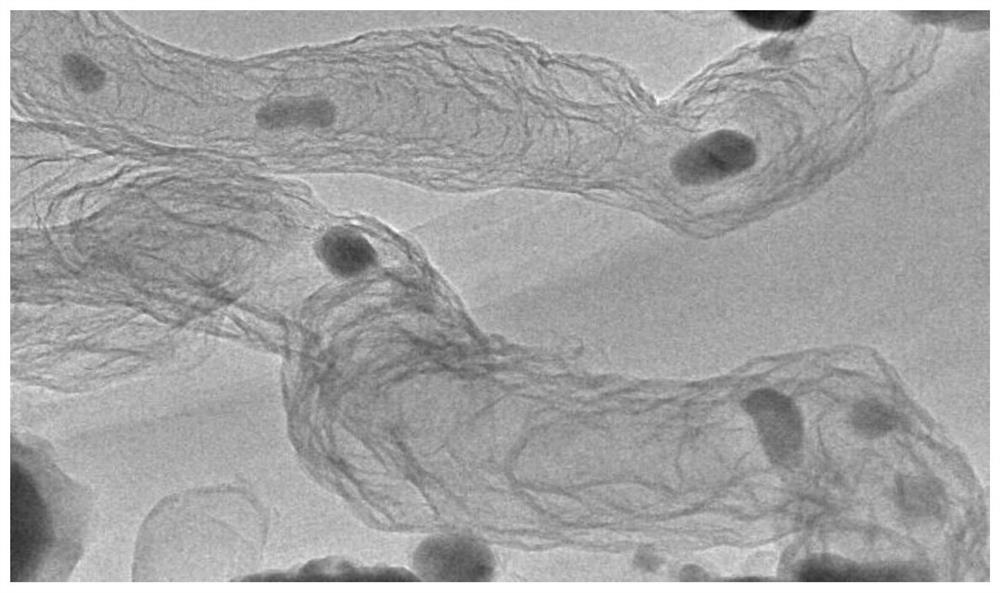
Image
Examples
Embodiment 1
[0039] 1. The preparation of nitrogen-doped iron nanotubes, the specific steps are as follows:
[0040] (1) put g-C 3 N 4 (0.8mol) and glucose (3.4mol) were placed in water, mixed evenly to form a uniform dispersion system, and ferric chloride (1.2mol) solution was added to the uniform dispersion system, and after ultrasonic reaction for 3 hours, it was transferred to a hydrothermal reaction kettle React at 120°C for 12h, after repeated centrifugation and washing, dry in a vacuum oven at 80°C for 6h.
[0041] (2) After the product obtained in step (1) was pyrolyzed at 800° C. for 2 h in a tube furnace in a nitrogen (80 mL / min) atmosphere, the core-shell FeN nanotube catalytic material could be obtained.
[0042] 2. Apply the obtained core-shell nitrogen-doped iron metal nanomaterials to electrocatalytic denitrification
[0043] The obtained FeN nanotube catalytic material (2 mg): carbon black: polyvinylidene fluoride (PVDF) was coated on a nickel mesh (1.2 cm × 1 cm) accord...
Embodiment 2
[0053] The preparation of nitrogen-doped iron nanotubes and the method for catalytic reduction of nitrate are as in Example 1, except that the doping amount of Fe in the process of preparing the FeN catalyst is 5wt%, and the nitrogen selectivity of the prepared catalyst FeN-5 is 50 %, the removal rate of nitrate is 62%.
Embodiment 3
[0055] The preparation of nitrogen-doped iron nanotubes and the method of catalytic reduction of nitrate are as in Example 1, except that the doping amount of Fe in the process of preparing FeN catalyst is 35wt%, and the nitrogen selectivity of the prepared catalyst FeN-35 is 86 %, the removal rate of nitrate is 91%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com