Synchronous analysis device, preparation method and application of bisphenol A and halogenated derivative thereof
An analysis device and a simultaneous analysis technology, which is applied in the directions of measuring devices, analysis materials, material excitation analysis, etc., can solve problems such as the inability to detect bisphenol A halogenated derivatives simultaneously and quickly, and achieve rapid adsorption, high-sensitivity detection, and large-scale The effect of specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
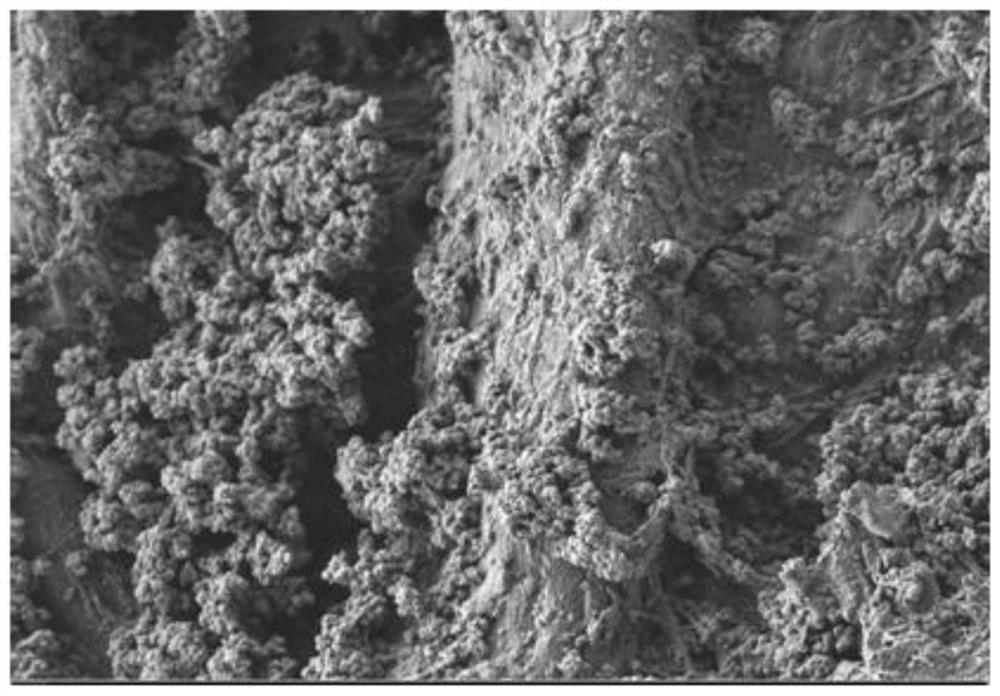
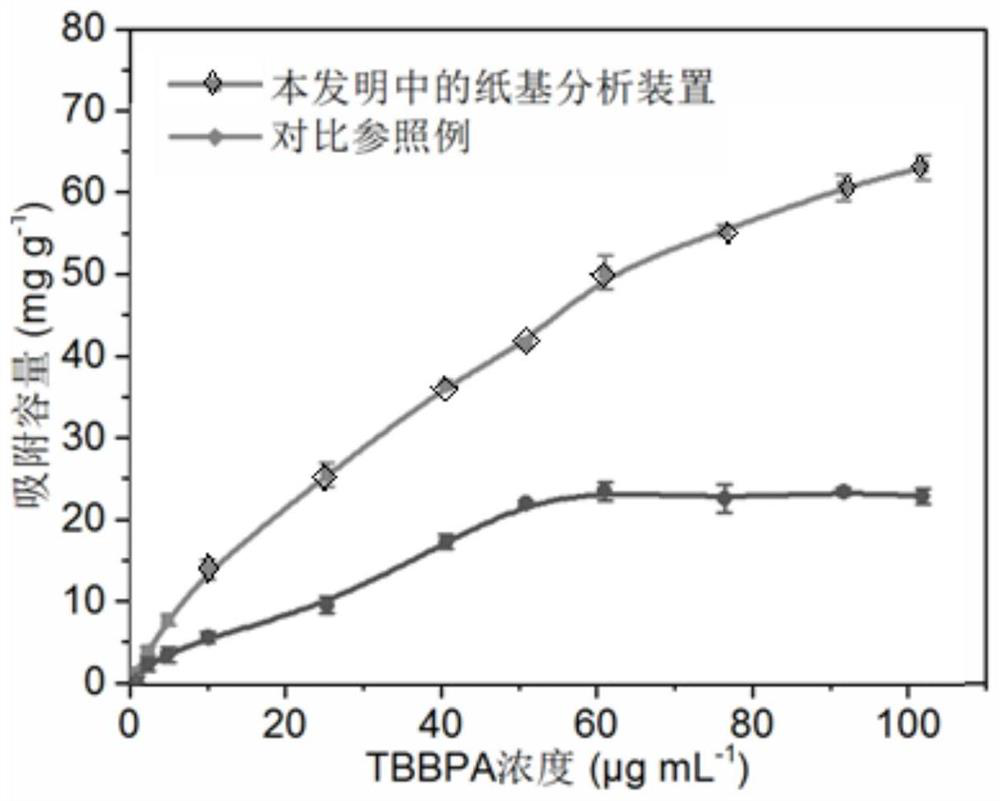
[0058] This embodiment provides a simultaneous analysis device for bisphenol A and its halogenated derivatives, which includes: a paper substrate and a composite material modified on the surface of the paper substrate, wherein the composite material includes UiO-66-NH 2 and a molecularly imprinted polymer, wherein the molecularly imprinted polymer is formed by using tetrabromobisphenol A as a template molecule, 3-aminopropyltriethoxysilane as a functional monomer, and tetraethyl orthosilicate as a crosslinking agent. The molecularly imprinted polymer can adsorb bisphenol A and / or its halogenated derivatives.
[0059] Wherein, the paper base can be Whatman filter paper. The analysis device can be, for example, a paper strip of 10×0.5 cm, which is easy to carry and use.
[0060] In this example, the metal-organic framework UiO-66-NH 2 Can be replaced by NH 2 -MIL-101(Fe), NH 2 - MIL-125(Ti) or ZIF-8.
Embodiment 2
[0062] This example provides a method for preparing a simultaneous analysis device for bisphenol A and its halogenated derivatives, see Figure 12 , which includes:
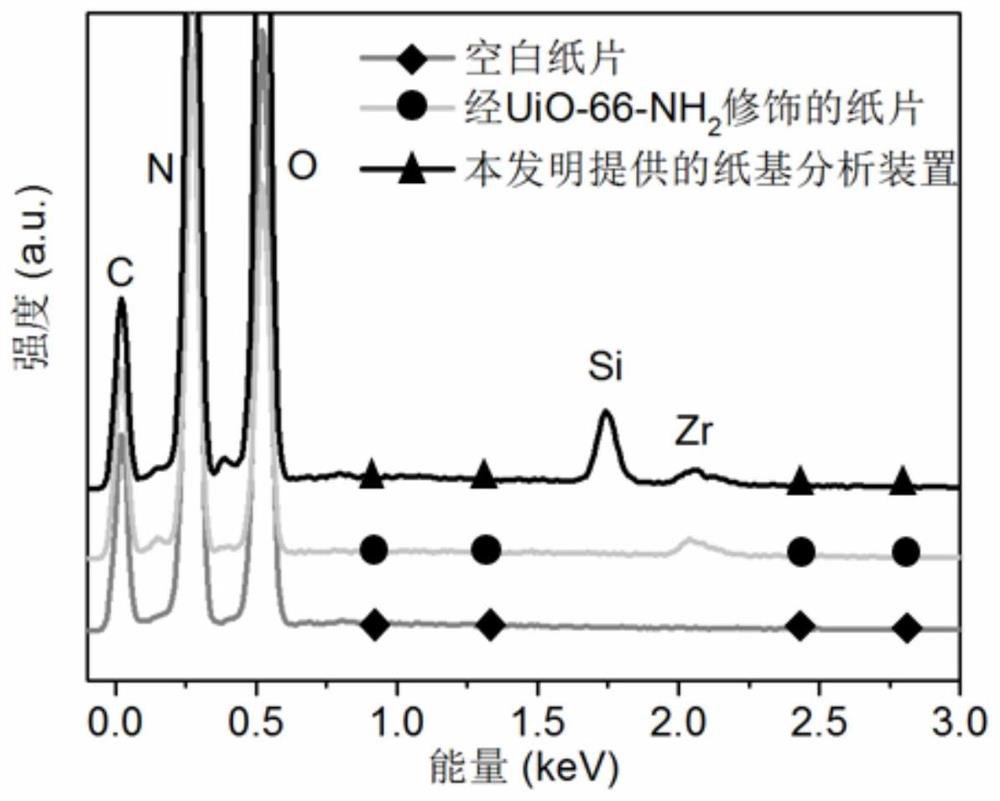
[0063] (1) 5.4mmol of zirconium chloride (ZrCl 4 ) was dissolved in the mixed solution of 10mL concentrated hydrochloric acid and 50mL dimethylformamide (DMF), ultrasonic 10min, obtained dissolved ZrCl 4 Solution A; Dissolve 7.5mmol of 2-aminoterephthalic acid in 100mL DMF, and sonicate for 10min to obtain solution B in which the ligand is dissolved; Mix solution A and solution B, sonicate for 10min, and stir at 80°C for 12h , a coordination reaction occurs. After the reaction is completed, it is cooled and washed by centrifugation, and dried at 150°C for 5 hours to obtain the dried UiO-66-NH 2 powder.
[0064] (2) 100mg of UiO-66-NH 2 The powder was added to 40mL ethanol, ultrasonicated for 30min to form a uniform dispersion, and the UiO-66-NH 2 embellished on Whatman paper substrates (10 × 10 cm in size), ...
Embodiment 3
[0068] This embodiment provides the application of an analysis device for simultaneous detection of bisphenol A and its halogenated derivatives. see Figure 12 , which includes:
[0069] (1) Obtain the extract of the substance to be detected: Weigh 100 mg of the dust of the substance to be detected into a 10 mL glass centrifuge tube, add 3 mL of the mixed extract of n-hexane and acetone, wherein the volume ratio of n-hexane and acetone is 3 :1. Sonicate for 10 minutes, vortex for 2 minutes, and then centrifuge at 5000 rpm for 10 minutes to obtain the supernatant. The above steps were repeated three times, the supernatants obtained three times were combined and then dried by nitrogen blowing, 0.1 mL of methanol was added to make up to volume, and stored at 4°C for future use.
[0070] (2) Add 10 μL of dust extract solution dropwise to one end of the analysis device prepared in Example 2, dry at room temperature, and remove unadsorbed impurities with water droplets at the pla...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com