Preparation method and application of iron-zinc vanadate ion battery positive electrode material
A technology for zinc-ion batteries and positive electrode materials, applied in battery electrodes, positive electrodes, secondary batteries, etc., can solve problems such as poor cycle stability of zinc-ion batteries, and achieve excellent zinc-ion battery performance, high rate performance, and uniform size Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] (1) Take 4mlH 2 o 2 Add to 40ml deionized water, weigh 2mmol of V 2 o 5 (0.364g) was added to the above solution, stirred in a water bath (300rpm) at 40°C until dissolved, and then weighed 0.2mmol of Fe(NO 3 ) 3 9H 2 Add O (0.0808g) to it, add 0.5g PVP after stirring for five minutes, and continue magnetic stirring for 30 minutes;
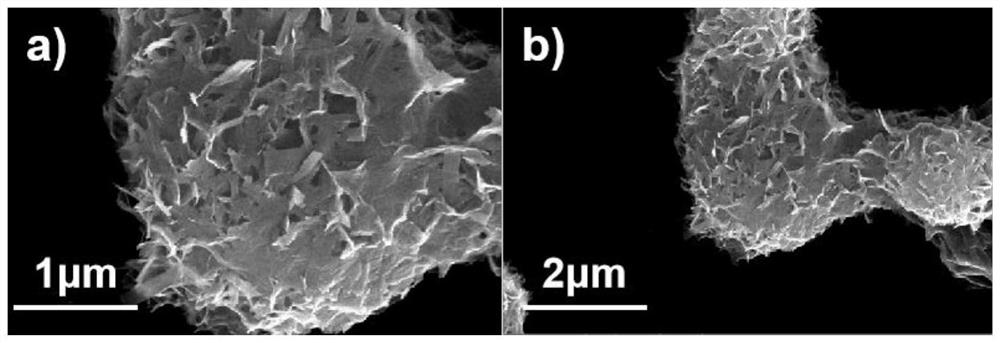
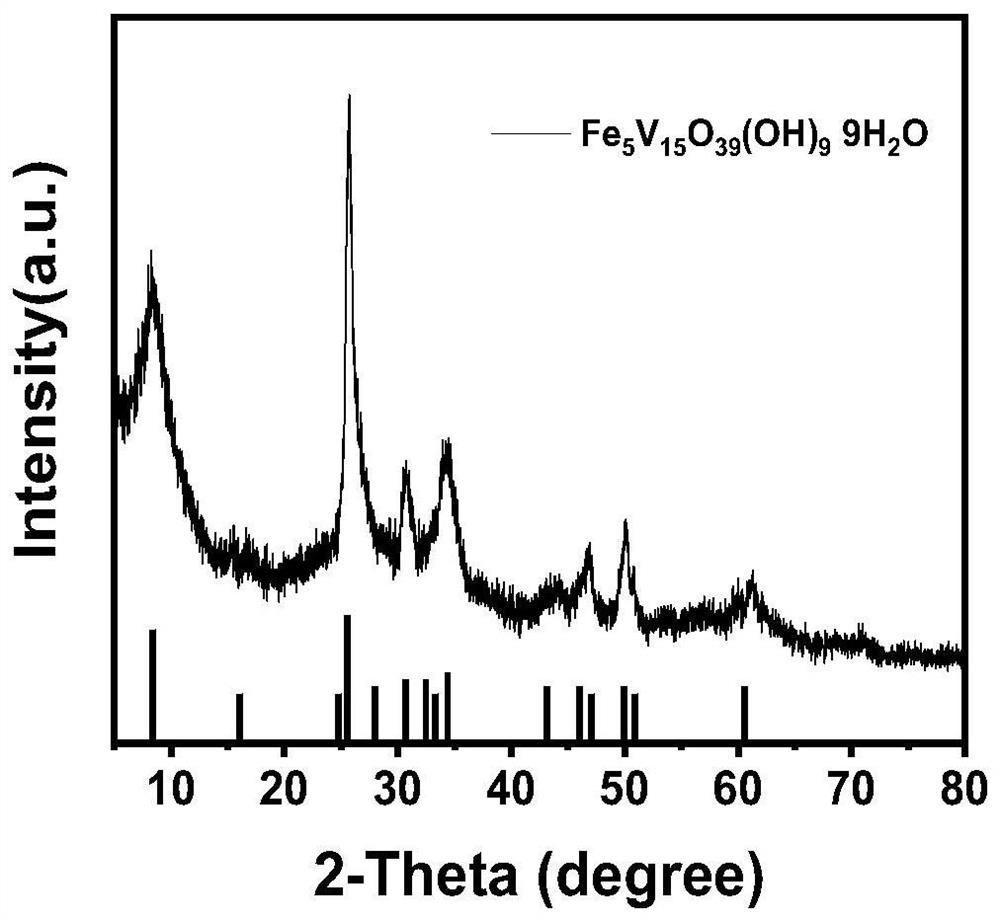
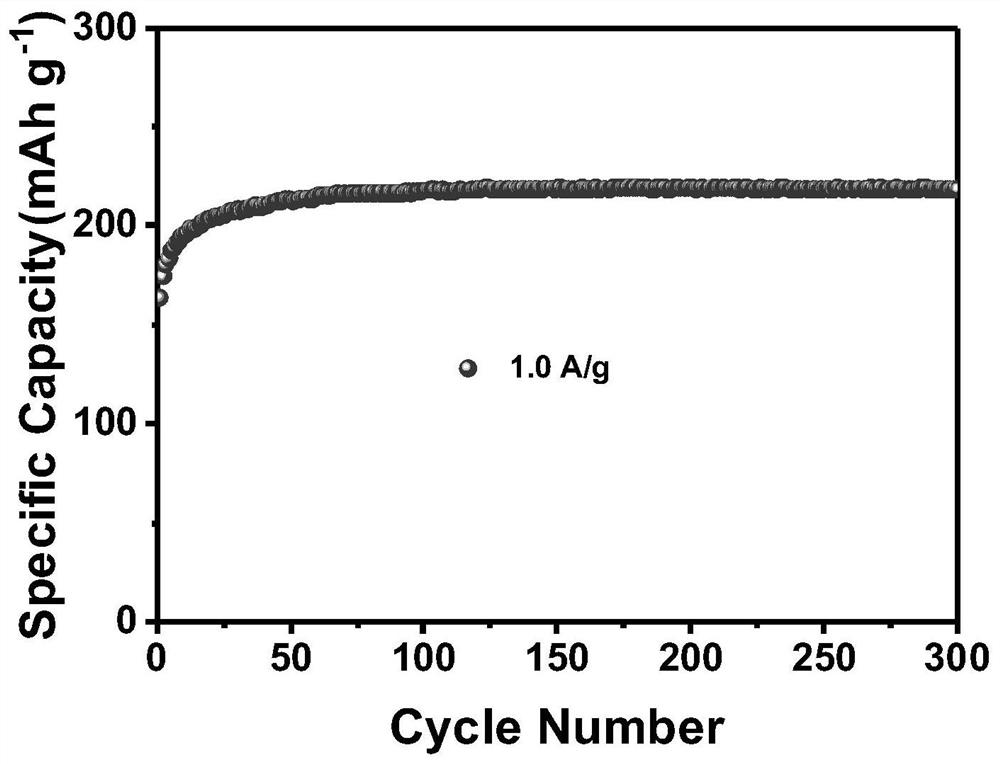
[0030] (2) Transfer the above mixed solution to a 50ml reaction kettle, and react at 180°C for 12h. After the reaction, the product was taken out and centrifuged and washed three times. The washing solvent was deionized water. The washed product was placed in a vacuum oven at 60°C for 12 hours to obtain the iron vanadate material; the SEM images of the iron vanadate material at different magnifications are as follows figure 1 Shown; The XRD pattern of this ferric vanadate material is as figure 2 show; combine figure 1 (a-b) It can be seen that the particle size of the iron vanadate material is 2 μm, which is determined by figure ...
Embodiment 2
[0035] (1) Take 1mlH 2 o 2 Add to 40ml deionized water, weigh 0.4mmol of NH 4 VO 3 Add to the above solution, stir in a water bath (300rpm) at 40°C until dissolved, then weigh 0.04mmol of FeCl 3 Add it, add 0.04g PVP after stirring for five minutes, and continue magnetic stirring for 30 minutes;
[0036] (2) Transfer the above mixed solution to a 50ml reactor and react at 100°C for 10h. After the reaction, the product was taken out and centrifuged and washed three times. The washing solvent was deionized water, and the washed product was placed in a vacuum oven at 60°C for 12 hours to obtain an iron vanadate material;
[0037] (3) Use the above-mentioned ferric vanadate material as the active material of the positive electrode material of zinc ion battery, grind it with carbon black and PVDF at a ratio of 8:1:1, add NMP as a solvent, grind it for 30 minutes, and use a scraper to scrape it on the Ti foil. Dry in a vacuum oven at 60°C;
Embodiment 3
[0041] (1) Take 10mlH 2 o 2 Add to 40ml deionized water, weigh 4mmol of NH 4 VO 3 Add to the above solution, stir in a water bath (300rpm) at 40°C until dissolved, then weigh 0.4mmol of FeCl 3 Add it, add 0.6g DTAB after stirring for five minutes, and continue magnetic stirring for 30 minutes;
[0042](2) Transfer the above mixed solution to a 50ml reactor and react at 200°C for 30h. After the reaction, the product was taken out and centrifuged and washed three times. The washing solvent was deionized water, and the washed product was placed in a vacuum oven at 60°C for 12 hours to obtain an iron vanadate material;
[0043] (3) Use the above-mentioned ferric vanadate material as the active material of the positive electrode material of zinc ion battery, grind it with carbon black and PVDF at a ratio of 8:1:1, add NMP as a solvent, grind it for 30 minutes, and use a scraper to scrape it on the Ti foil. Dry in a vacuum oven at 60°C;
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com