Method for reducing byproduct chlorotoluene in benzyl chloride production process
A production process, technology of benzyl chloride, applied in the field of photocatalytic reaction of toluene and chlorine to produce benzyl chloride, can solve the problem of reduced activity of substitution reaction, achieve the effect of improving selectivity, reducing heat loss, and reducing deep substitution reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
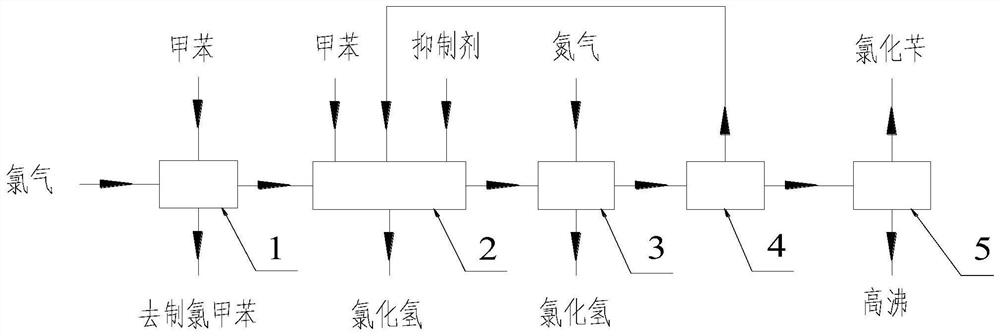
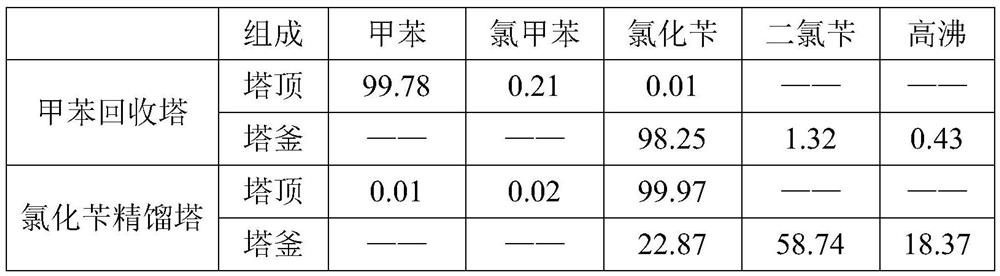
[0037] Add tributyl phosphate into a photochlorinated glass reaction kettle equipped with an ultraviolet light source according to the mass ratio of 0.05% to toluene (water content 29ppm), mix the materials evenly and preheat to 90°C, and pass the chlorine gas after metal removal into the In the light chlorination reaction kettle, the chlorination reaction temperature is controlled at 90°C during the reaction process, and the hydrogen chloride produced by the reaction is removed from the tail gas treatment process. When the conversion rate of toluene reaches 24.8%, the reaction liquid is added to the aeration kettle and nitrogen is introduced. After 30 minutes of aeration, the acid value of the material was 196ppm, and the temperature dropped to 81.5°C. The material was added to the toluene recovery tower, and the rectification vacuum was controlled to be -0.09MPa. A toluene stream containing a small amount of o- / p-chlorotoluene was obtained at the top of the tower, and the tolu...
Embodiment 2
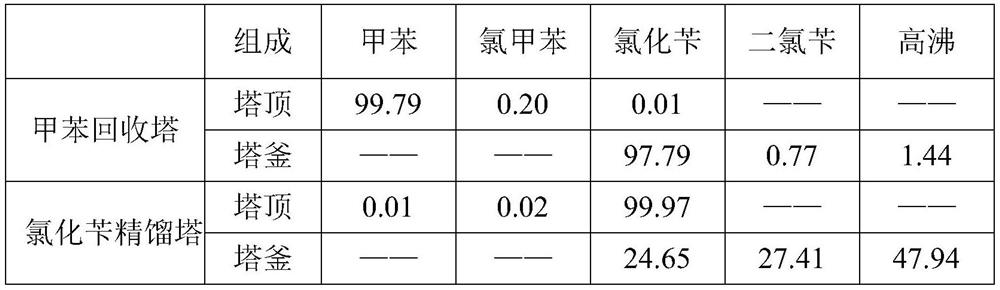
[0041] Add tributyl phosphate and phenylethylene glycol amine to a photochlorinated glass reactor equipped with an ultraviolet light source at a mass ratio of 0.20% and 0.05% to toluene (water content 29ppm) respectively, mix the materials evenly and preheat to 105°C , pass the chlorine gas after metal removal into the photochlorination reaction kettle, control the chlorination reaction temperature to be 105°C during the reaction, and remove the tail gas treatment process for the hydrogen chloride produced by the reaction. When the toluene conversion rate reaches 20.3%, the reaction solution is Add it into the aeration kettle, and pass nitrogen gas into the aeration tank for 30 minutes, the acid value of the material is 177ppm, the temperature drops to 93.3°C, the material is added to the toluene recovery tower, the rectification vacuum is controlled to -0.08MPa, and a small amount of ortho is obtained at the top of the tower. / toluene flow of p-chlorotoluene, the toluene recov...
Embodiment 3
[0045] Add triethanolamine to toluene (water content 29ppm) in a mass ratio of 0.50% to a photochlorination glass reactor equipped with an ultraviolet light source, mix the materials evenly and preheat to 97°C, and pass the chlorine gas after metal removal into the photochlorination glass reactor. In the chemical reaction kettle, the chlorination reaction temperature is controlled at 97°C during the reaction process, and the hydrogen chloride generated by the reaction is removed from the tail gas treatment process. When the conversion rate of toluene reaches 22.4%, the reaction solution is added to the aeration kettle, and nitrogen is aerated. After 30 minutes, the acid value of the material was 185ppm, and the temperature dropped to 89.6°C. The material was added to the toluene recovery tower, and the rectification vacuum was controlled to be -0.085MPa. A small amount of o- / p-chlorotoluene was obtained at the top of the tower. Tower top material joins in the chlorination react...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com