A kind of method for preparing adenovirus vector vaccine by perfusion culture technique
A vector vaccine and culture method technology, applied in the field of biological products, can solve the problems of low cell density yield, limited growth, low cell density, etc., and achieve the effects of increasing single cell yield and virus titer.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Example 1 Confirmation of technical parameters for culturing 293 cells by perfusion method.
[0074] Perfusion process 1: After recovery, 293 cells were expanded and inoculated into the fermenter. When the cell density reached 1×10 6 Perfusion was started after cells / mL, and the perfusion rate was 2VVD. Continue to grow the cell density to 5×10 6 cells / mL, the perfusion rate was adjusted to 3VVD.
[0075] Perfusion process 2: After recovery, 293 cells were expanded and inoculated into the fermenter. When the cell density reached 1×10 6 / mL or 5×10 6 cells / mL and start perfusion. Throughout the culture process, the perfusion rate was maintained constant at 1VVD or 3VVD.
[0076] Perfusion process 3: After 293 cells were recovered, they were expanded and inoculated into the fermenter. When the cell density reached 1×10 6 / mL or 5×10 6 cells / mL and start perfusion. Throughout the culture process, the perfusion rate was maintained constant at 2VVD or 4VVD.
[0077]...
Embodiment 2
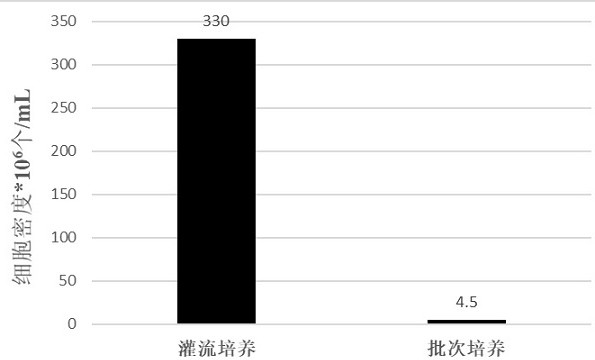
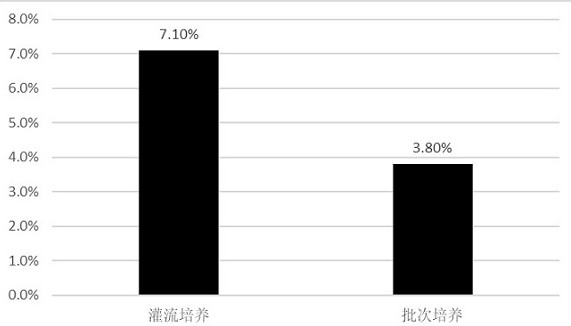
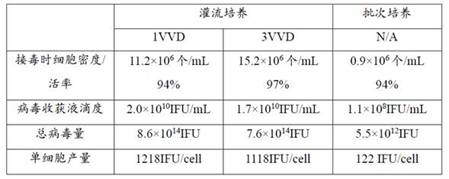
[0089] Example 2: Comparison of cell density and viability between the perfusion culture process and the batch process culture.
[0090] Experiment 1: Culture was carried out by adopting the conventional batch culture technology in this field.
[0091] Experiment 2: Using the perfusion process 1 of Example 1, that is, after the 293 cells were recovered, they were expanded and inoculated into the fermenter. When the cell density reached 1×10 6 Perfusion was started after cells / mL, and the perfusion rate was 2VVD. Continue to grow the cell density to 5×10 6 cells / mL, the perfusion rate was adjusted to 3VVD.
[0092] In experiments 1 and 2, other parameters (such as culture temperature, pH dissolved oxygen concentration, stirring speed, etc.) were basically the same during the culture process. The cell density and viability after the culture were detected, and the results are shown in Table 4.
[0093] Table 4 The viability and density of 293 cells cultured by batch process an...
Embodiment 3
[0096] Example 3: Effect of glutamine on cell production in a perfusion process.
[0097] Experiment 1: After recovery of 293 cells, they were expanded and inoculated into fermenters. When the cell density reached 1×10 6 Perfusion was started after cells / mL, and the perfusion rate was 2VVD. Continue to grow the cell density to 5×10 6 cells / mL, the perfusion rate was adjusted to 3VVD. Glutamine was not added during the perfusion process.
[0098] Experiment 2: After recovery of 293 cells, they were expanded and inoculated into fermenters. When the cell density reached 1×10 6 Perfusion was started after cells / mL, and the perfusion rate was 2VVD. Continue to grow the cell density to 5×10 6 cells / mL, the perfusion rate was adjusted to 3VVD. Monitor the concentration of glutamine in the perfusion process, add glutamine, and maintain the concentration of glutamine to 2mM.
[0099] Experiment 3: After recovery, 293 cells were expanded and inoculated into fermenters, when the cel...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com