Preparation method of soluble chromium hydroxide
A technology of chromium hydroxide and solution, applied in chemical instruments and methods, chromium compounds, inorganic chemistry, etc., can solve the problems of high cost, harsh conditions, and poor working environment, and achieve low cost, mild reaction conditions, and easy precipitation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
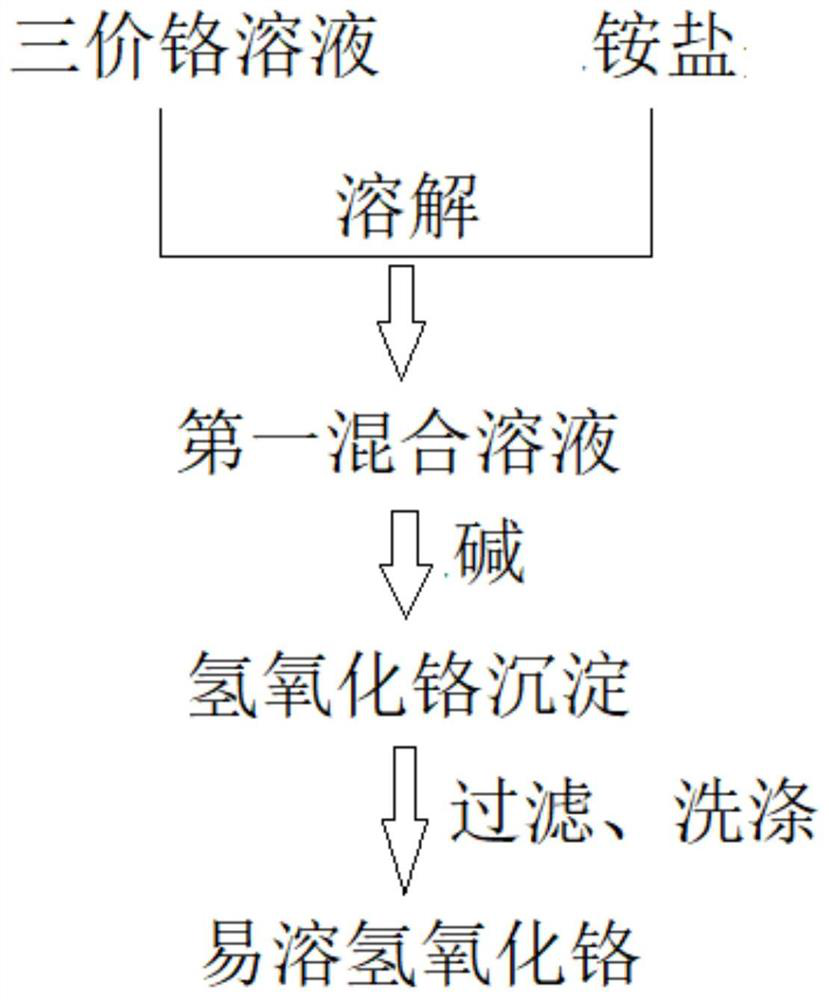
[0028] Such as figure 1 As shown, some embodiments of the present invention disclose a kind of preparation method of soluble chromium hydroxide, comprising the steps:
[0029] Step 1: adding ammonium salt to the trivalent chromium solution and dissolving it to obtain the first mixed solution;
[0030] Step 2: Under predetermined reaction conditions, alkali is added to the first mixed solution to obtain chromium hydroxide precipitation, and the ammonia released during the precipitation process can be absorbed and reused;
[0031] Step 3: Filter and wash the precipitated chromium hydroxide to obtain easily soluble chromium hydroxide, and the filtrate can directly enter the waste water treatment process.
[0032] Wherein, the ammonium salt is water-soluble and has a pH value not greater than 7; the ammonium salt can be one of ammonium sulfate, ammonium bisulfate, ammonium chloride, ammonium nitrate, and ammonium acetate. In some preferred embodiments, the anion of the ammonium ...
Embodiment 1
[0038] Chromium chloride solution 1000mL (where Cr 3+ =10g / L), add ammonium chloride 6.2g (n(NH 4+ ):n(Cr 3+) = 0.6), stirring and dissolving; adding sodium bicarbonate lye at a temperature of 20°C and a stirring speed of 200 to 300r / min, controlling the end point pH to be 6.0, and stirring and reacting for 30 minutes after adding alkali; filtering and washing to obtain soluble hydrogen Chromium oxide, the filtrate enters the wastewater treatment process. The obtained chromium hydroxide precipitate was placed in 0.1 mol / L HCl solution (1000 mL), stirred and dissolved at room temperature, and the required time was about 3 min.
Embodiment 2
[0040] Chromium sulfate solution 2000mL (where Cr 3+ =26g / L), add ammonium sulfate 132g (n(NH 4+ ):n(Cr 3+ )=2.0), stir and dissolve; add sodium carbonate lye at a temperature of 60°C and a stirring speed of 400 to 500r / min, control the end point pH to 7.0, and complete the stirring reaction for 15 minutes after adding alkali; filter and wash to obtain soluble hydroxide Chromium, the filtrate enters the wastewater treatment process. Place the obtained chromium hydroxide precipitate in 0.05mol / L H 2 SO 4 solution (2000mL), stirring and dissolving at room temperature, the required time is about 5min.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com