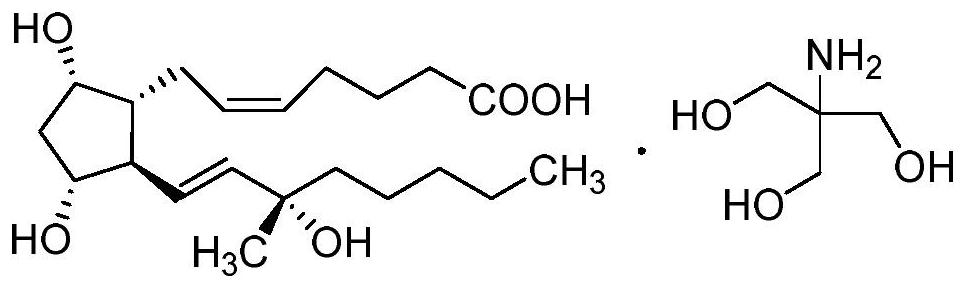
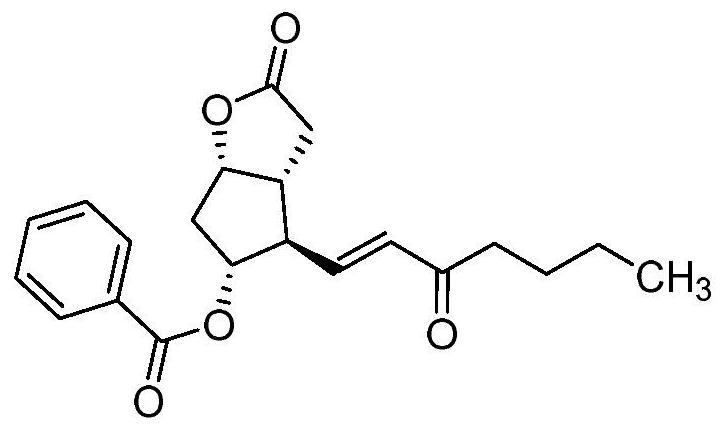
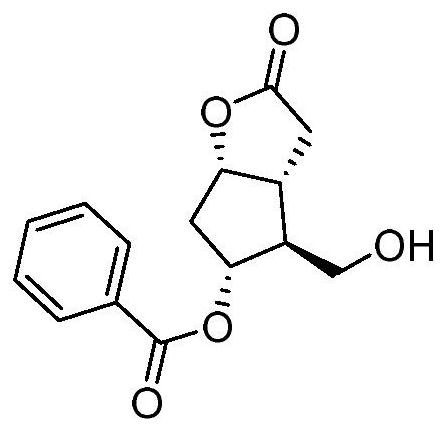
Carboprost tromethamine related impurity L-amyl 15-ketone and preparation method thereof
A technology of pentyl and triphenylphosphine, which is applied in the field of compound preparation to achieve the effects of high yield, high safety and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Add 57.0g of methyltriphenylphosphine bromide and 300ml of tetrahydrofuran into a 1L four-neck flask, stir and cool down to -20°C to -15°C under nitrogen protection, and add 76.8ml of 2.5M n-butyllithium dropwise (the dropwise addition is completed in 30 minutes ), carry out the dehydrogenation reaction for 2 hours after the addition, maintain the temperature range of -20°C to -15°C, add 27.5g of ethyl valerate dropwise, continue the reaction for 3h after the addition, add 240ml of water to quench the reaction, separate liquid, water Layer was extracted twice with 200ml of ethyl acetate, the organic layers were combined, washed with 200ml of saturated sodium chloride, dried over anhydrous sodium sulfate, filtered with suction, and the filtrate was concentrated under reduced pressure to obtain an oil, which was left to precipitate a solid, and isopropyl ether was added 200ml of slurry was beaten for 2 hours, suction filtered, the filter cake was washed with 50ml of isopro...
Embodiment 2
[0037] Add 57.0g of methyltriphenylphosphine bromide and 300ml of tetrahydrofuran into a 1L four-neck flask, stir and cool down to -20°C to -15°C under nitrogen protection, and add 76.8ml of 2.5M n-butyllithium dropwise (the dropwise addition is completed in 30 minutes ), carry out the dehydrogenation reaction for 2 hours after the addition, maintain the temperature range of -20°C to -15°C, add 27.5g of ethyl valerate dropwise, continue the reaction for 3h after the addition, add 240ml of water to quench the reaction, separate liquid, water layer was washed twice with 200ml of ethyl acetate for extraction, the organic layers were combined, washed with 200ml of saturated sodium chloride, dried over anhydrous sodium sulfate, filtered with suction, the filtrate was concentrated under reduced pressure to obtain an oily substance, which was left to precipitate a solid, and isopropyl ether was added 200ml was beaten for 2h, suction filtered, the filter cake was washed with 50ml of is...
Embodiment 3
[0041] Add 57.0 g of methyltriphenylphosphine bromide and 300 ml of tetrahydrofuran into a 1L four-neck flask, stir and cool down to -20°C to -15°C under nitrogen protection, and add 22.0g of potassium tert-butoxide in batches (the addition is completed in 30 minutes), After the addition, carry out the dehydrogenation reaction for 2 hours, maintain the temperature range of -20°C to -15°C, add 27.5g of ethyl valerate dropwise, continue the reaction for 3h after the addition, add 240ml of water to quench the reaction, separate the liquid, and use the water layer Wash 2 times with 200ml of ethyl acetate for extraction, combine the organic layers, wash with 200ml of saturated sodium chloride, dry with anhydrous sodium sulfate, filter with suction, concentrate the filtrate under reduced pressure to obtain an oil, place it to precipitate a solid, add 200ml of isopropyl ether for beating After 2 hours, filter with suction, wash the filter cake with 50 ml of isopropyl ether, and dry to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com