Pig-derived ACE inhibitory activity polypeptide, pharmaceutical composition or food and application
A technology of active polypeptide and inhibitory activity, applied in blood pressure lowering drugs or food, porcine-derived ACE inhibitory active polypeptide and pharmaceutical composition or food and application, in the field of preparing ACE inhibitor, can solve the problem of short action time and easy blood pressure. Rebound, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
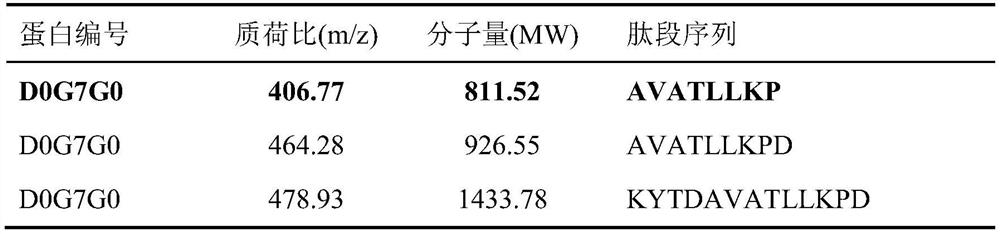
[0024] Example 1 Screening and Identification of Polypeptide AVATLLKP
[0025] A method combining LC-MS / MS with Shotgun proteomics technology was used. Pig limb bones are used as raw materials, and the pig bone extract is obtained after decoction, ethanol precipitation, acid precipitation, and alkali precipitation. The extract is subjected to LC-MS / MS analysis after centrifugation, ultrafiltration, and desalination. Structure-activity relationship features to screen peptides that have an inhibitory effect on ACE.
[0026] The specific method is as follows:
[0027] Sample preparation: (1) Extraction: fresh pig limb bones were washed, crushed, weighed, added water according to the ratio of material to liquid 1:2 (w / v), extracted under 1.2MPa hot pressure for 1.5h, filtered, repeated extraction once, combined filtrate. (2) Degreasing: the filtrate was cooled at 4°C for 36 hours, and the upper layer of fat was removed. (3) Ethanol precipitation: concentrate in vacuo, add etha...
Embodiment 2
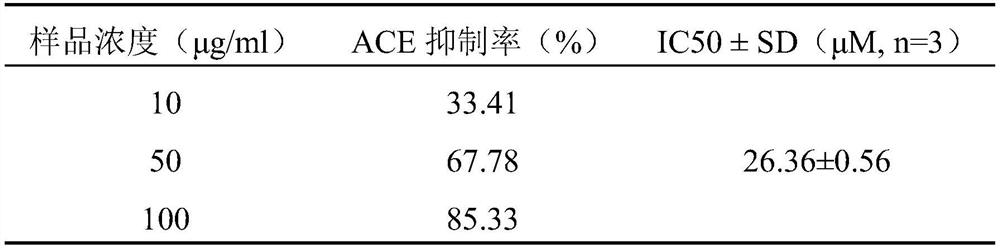
[0033] Example 2 Detection of ACE Inhibitory Activity of Polypeptide AVATLLKP
[0034] principle
[0035] N-[3-(2-furyl)acryloyl]-L-phenylalanyl-glycyl-glycine (FAPGG, λmax=340nm, ε=2270M -1 cm -1 , molecular weight 399.40) can be enzymatically hydrolyzed by ACE into N-[3-(2-furyl)acryloyl]-L-phenylalanyl (FAP, λmax=340nm, ε=1512M -1 cm -1 ) and glycyl-glycine (GG, no absorption at 340nm), so it can be used as the mimic substrate of ACE. The absorbance value of 1mM FAPGG completely converted into FAP and GG is 0.758, and the inhibition rate can be calculated according to the change value of absorbance at 340nm.
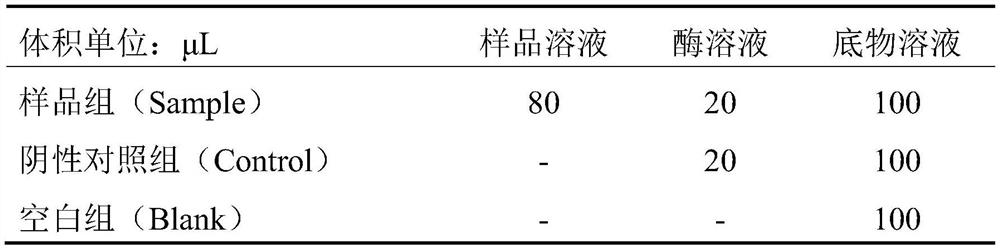
[0036] reaction system
[0037] (1) Buffer solution: 0.1M PBS buffer solution (pH 8.2, containing 300mM NaCl)
[0038] (2) Substrate solution: FAPGG solution with a concentration of 1.6 mM was prepared using the above buffer solution.
[0039] (3) Enzyme solution: ACE was prepared into a 0.2 U / mL solution using the above buffer solution.
[0040] (4) Sample solu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com