Antigen and indirect ELISA test kit for identification of Mycoplasma bovis vaccine strain or field strain infection
A detection kit, a technology for Mycoplasma bovis, which is used in biological tests, measuring devices, immunoassays, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
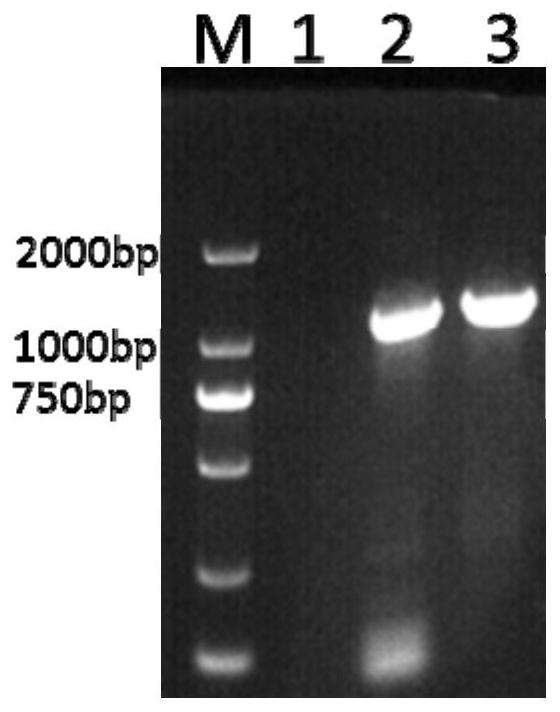
[0061] Example 1 Membrane protein (P275) gene, leucyl aminopeptidase (pepA) gene fragment PCR amplification
[0062] 1. Cultivation of Mycoplasma bovis strains: Inoculate the Mycoplasma strains isolated from diseased cattle by our company into PPLO liquid medium at 37°C and 5% CO 2 Cultivated in an incubator;
[0063] 2. Genomic DNA extraction: extract the genome according to the method provided by the Mycoplasma Genomic DNA Extraction Kit;
[0064] 3. Target gene amplification: Amplification system is 50 μL system: Taq enzyme (1000u / mL) 2 μL, 10×BUFFER 5 μL, dNTP (10 mM) 2 μL, upstream primer (10 μM) 2 μL, downstream primer (10 μM) 2 μL, template 10 μL , ddH 2 O 27 μL. PCR reaction conditions: pre-denaturation at 95°C for 5 minutes; 35 cycles of denaturation at 95°C for 30 seconds, annealing at 52°C for 30 seconds, and extension at 72°C for 1 minute; extension at 72°C for 15 minutes; storage at 4°C.
[0065] 4. Purification of the target gene: PCR product gel electrophore...
Embodiment 2
[0066] Embodiment 2 connection and conversion
[0067] 1. Plasmid pET-30a (+), membrane protein (P275) gene, and leucyl aminopeptidase (pepA) gene were subjected to KpnI and BamHI double enzyme digestion;
[0068] 1.1 Perform 1% agarose gel electrophoresis on the digested product, recover the target band from the gel and purify it;
[0069] 1.2 According to the method steps provided by the T4 connection kit, connect the plasmid with the p275 and pepA genes;
[0070] 1.3 Kanamycin (100ml / L) solid medium screening P275-pET-30a (+), pepA-pET-30a (+) transformed into Escherichia coli BL21 positive strain;
[0071] 1.4 Identification of P275-pET-30a(+), pepA-pET-30a(+) recombinant prokaryotic expression vectors by double enzyme digestion.
[0072] 2. Prokaryotic expression and purification of P275-pET-30a(+), pepA-pET-30a(+) proteins
[0073] 2.1 Pick a single colony of Escherichia coli BL21 containing P275-pET-30a (+), pepA-pET-30a (+) expression vector in LB solid medium (cont...
Embodiment 3E
[0080] Embodiment 3ELISA detects bovine serum
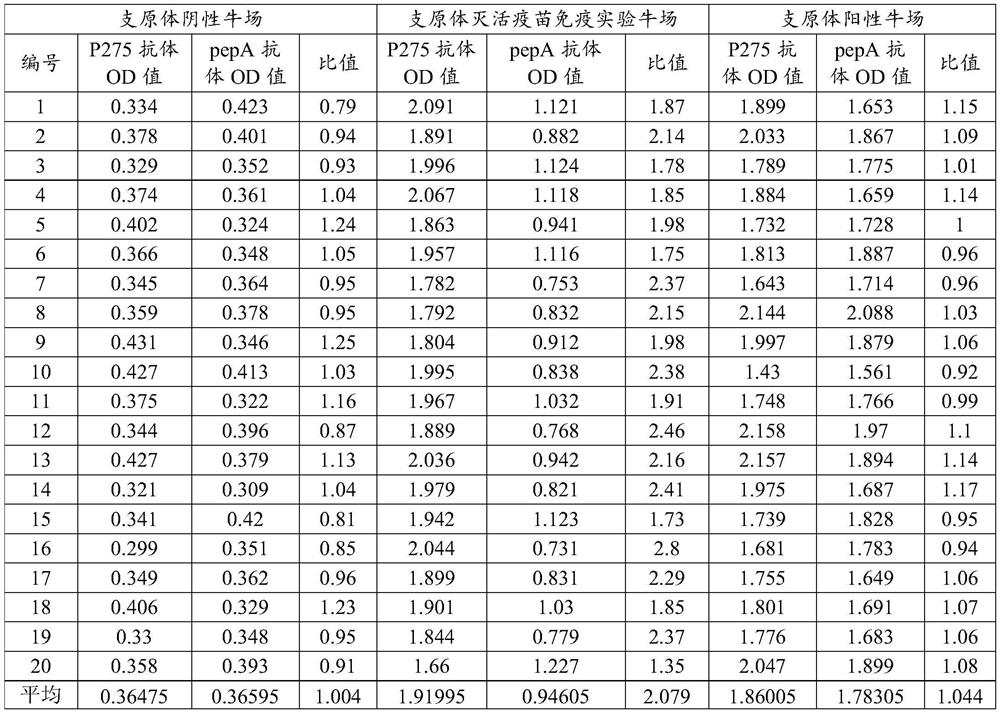
[0081] Randomly select 20 cattle from each of the mycoplasma-negative cattle farm, the inactivated vaccine immunization experiment cattle farm, and the mycoplasma-positive cattle farm. Follow the indirect ELISA method to detect the OD of P275 protein and pepA protein antibody in serum 450nm values (see Table 1 for the results).
[0082] 1. Take 5ml of blood from the neck of the cow, centrifuge at 1000g for 20min at 37°C for 2h, and store the supernatant at 4°C;
[0083] 2. Coating: Dilute P275 expression protein concentration to 50 μg / ml and pepA protein concentration to 80 μg / ml with coating diluent (PBS pH7.0), respectively add to different microwells of the ELISA plate, 100 μl per well, 4 ℃ 24h;
[0084] 3. Blocking: Discard the coating solution, add 250 μl of 5% protamine to each well, and keep at 37°C for 1 hour;
[0085] 4. Plate washing: Discard the blocking solution, add washing solution to fill the wells, vibrate f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com