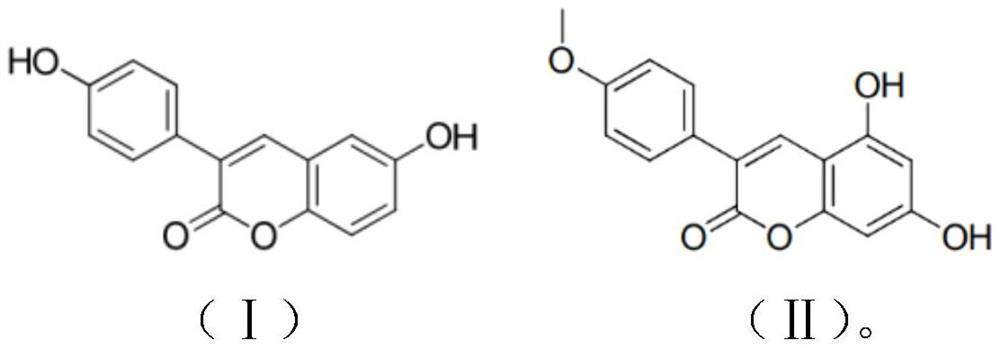
Application of 3-aryl coumarin compound
An aryl coumarin and compound technology, which is applied in the directions of active ingredients of heterocyclic compounds, drug combinations, metabolic diseases, etc., can solve the problems of poor therapeutic effect, large side effects, and single action target.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Determination of the toxicity of two compounds to cells
[0028] (1) Human aortic vascular smooth muscle cells of 2-8 generations were cultured, a single cell suspension was made into a culture medium containing 10% fetal bovine serum, and 5000 cells per well were inoculated into a 96-well plate with a volume of 200 ul per well.
[0029] (2) Set up 7 groups, namely: normal group, Ⅰ-6.25ug, Ⅰ-12.5ug, Ⅰ-25ug, Ⅱ-6.25ug, Ⅱ-12.5ug, Ⅱ-25ug. Six replicate wells were set up for each drug group. The same as the general culture conditions, culture 24h, 48h.
[0030] (3) After the incubation, add 20ul of MTT solution (5mg / ml, prepared with PBS) to each well.
[0031] Continue to incubate for 4 hours, terminate the culture, carefully aspirate and discard the culture supernatant in the well, for suspension cells, centrifuge and then aspirate and discard the culture supernatant in the well. Add 150ul DMSO to each well and shake for 10 minutes to fully melt the crystals....
Embodiment 2
[0036] Example 2: Calcification induction of human vascular smooth muscle cells
[0037] (1) Preparation of calcification induction solution
[0038] Weigh 20 mg of bovine serum albumin and dissolve it in 50 mmol / L PBS (pH7.4) to prepare a buffer solution of bovine serum albumin in PBS (pH7.4) with a final concentration of 10 mg / mL. Weigh 79.2 mg of glucose, dissolve it in 2 ml of distilled water, and prepare a glucose solution with a final concentration of 0.2 mol / L. The above two solutions were mixed and filtered, and 0.02% double antibody was added to prevent bacterial growth. The above mixed solution was incubated at 37°C for 14 days, and the excitation wavelength and emission wavelength were set to 350nm and 420nm respectively using a fluorescence spectrophotometer, and the measured fluorescence intensity of AGE was as follows:
[0039]
[0040] (2) Calcification induction of human aortic smooth muscle
[0041] Human aortic vascular smooth muscle cells (HAVSMCs) wer...
Embodiment 3
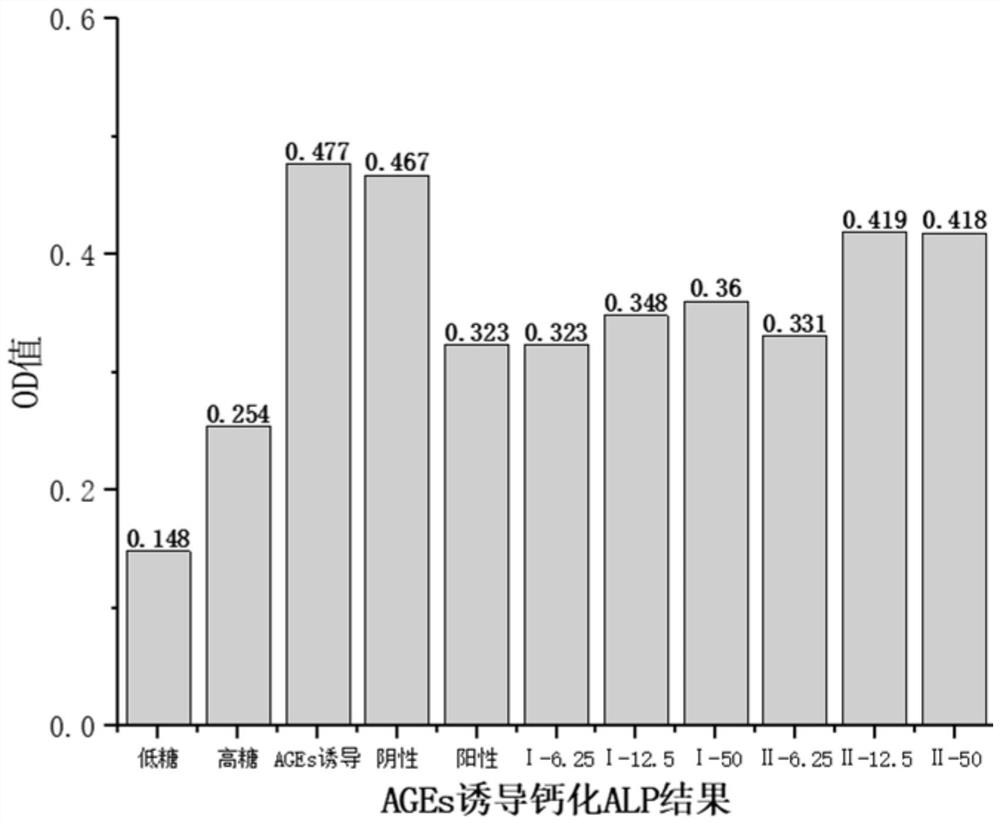
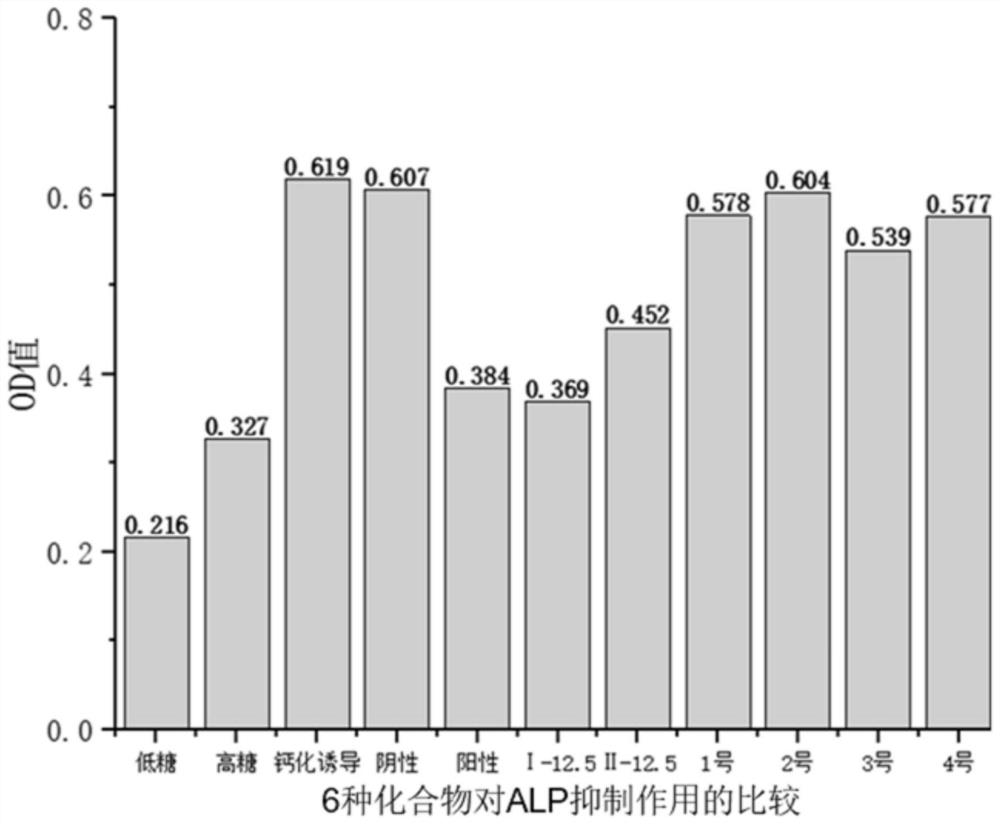
[0044] Example 3: Determination of the inhibitory effect of two compounds on the activity of alkaline phosphatase in calcified cells
[0045] 1. Determination principle of alkaline phosphatase activity in vitro
[0046] Alkaline phosphatase is widely distributed in the bones, intestines, kidneys, liver, placenta and other tissues of the human body. Under alkaline conditions, Para-nitrophenyl phosphate (pNPP) can generate para-nitrophenol under the action of alkaline phosphatase. Para-nitrophenol is a yellow product under alkaline conditions, and the absorbance can be detected at 400-415nm. The deeper the yellow color of the product, the higher the alkaline phosphatase activity and the higher the absorbance value, otherwise the lower the enzyme activity.
[0047] 2. Preparation of reaction solution
[0048] (1) Reagent preparation: Take out all reagents and return to room temperature for use.
[0049] (2) Preparation of chromogenic substrate solution: Take a tube of chromog...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com