Preparation method and application of calcite-rhodochrosite solid solution
A rhodochrosite and solid solution technology, applied in chemical instruments and methods, manganese nitrate, inorganic chemistry, etc., can solve the problems of safety hazards, manganese secondary pollution, low crystallinity of manganese slag, etc., and achieve low cost and easy control.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
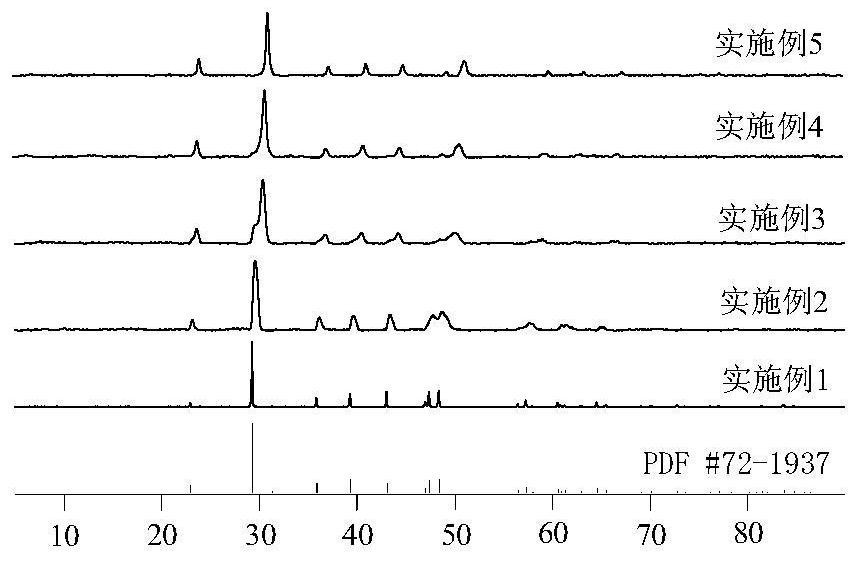
Image
Examples
Embodiment 1
[0017] At room temperature, under the condition of nitrogen protection, take 1000mL 0.5mol / L ammonium bicarbonate solution in a 1000mL polyethylene bottle, place it on a magnetic stirrer and stir at 20°C 700rmp for 3min; then mix 50mL 2.00mol / L calcium nitrate and 0mL2 .00mol / L manganese nitrate mixed solution is added to the polyethylene bottle at a speed of 10mL / min, and the reaction is stopped after reaching 10min, and the manganese nitrate / (calcium nitrate+manganese nitrate) molar ratio of 0.0 can be obtained. Calcite-rhodochrosite mixed crystal solid solution. The prepared reactant was subjected to solid-liquid separation, and the solid-phase precipitate was washed three times with 50 mL of ultrapure water and once with 50 mL of absolute ethanol, and then dried in a blast oven at 90°C for 24 hours to obtain a highly crystalline calcite solid solution.
Embodiment 2
[0019] At room temperature and under the condition of nitrogen protection, take 1000mL 0.5mol / L ammonium bicarbonate solution in a 1000mL polyethylene bottle, place it on a magnetic stirrer and stir at 20°C 700rmp for 3min; then mix 40mL 2.00mol / L calcium nitrate and 10mL2 .00mol / L manganese nitrate mixed solution is added to the polyethylene bottle at a speed of 10mL / min, and the reaction is stopped after reaching 10 minutes, and the manganese nitrate / (calcium nitrate+manganese nitrate) molar ratio of 0.2 can be obtained. Calcite-rhodochrosite mixed crystal solid solution. The prepared reactant was subjected to solid-liquid separation, and the solid-phase precipitate was washed three times with 50mL ultrapure water and once with 50mL absolute ethanol, and then dried in a blast drying oven at 90°C for 24 hours to obtain a highly crystalline calcite-rhodochrosite solid solution .
[0020] Leaching test: degassed ultrapure water, dissolved at 25°C for 2400h, manganese leaching ...
Embodiment 3
[0024] At room temperature, under the condition of nitrogen protection, take 1000mL 0.5mol / L ammonium bicarbonate solution in a 1000mL polyethylene bottle, place it on a magnetic stirrer and stir at 20°C 700rmp for 3min; then mix 25mL 2.00mol / L calcium nitrate and 25mL2 Add 0.00mol / L manganese nitrate mixed solution into the polyethylene bottle at a rate of 10mL / min, stop the reaction after reaching 10min, and then the calcite-rhodochrosite with manganese nitrate / (calcium nitrate+manganese nitrate) molar ratio of 0.5 can be obtained mixed crystal solid solution. The prepared reactant was subjected to solid-liquid separation, and the solid-phase precipitate was washed three times with 50mL ultrapure water and once with 50mL absolute ethanol, and then dried in a blast drying oven at 90°C for 24 hours to obtain a highly crystalline calcite-rhodochrosite solid solution .
[0025] Leaching test: degassed ultrapure water, dissolved at 25°C for 2400 hours, manganese leaching concent...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com