Gambogic acid nano preparation based on hydrophobic prodrug and capable of improving long circulation
A nano-preparation, gambogic acid technology, applied in the field of medicine, can solve problems such as leakage, low drug loading efficiency, and drug crystallization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: the preparation of oleyl bromide
[0042]
[0043] Add 1 g of oleyl alcohol to the reaction vessel, add 20 ml of anhydrous dichloromethane to dissolve, then add 504.1 mg of phosphorus tribromide, stir at room temperature for 30 minutes, and separate by silica gel column chromatography to obtain 598.9 mg of a white oily substance.
[0044] of the substance 1 HNMR and MS data are as follows:
[0045] 1 HNMR (CDCl 3,300MHz)δ: 5.46–5.31(m,2H,9-H,10-H),3.43(t,J=6.9Hz,2H,1-H),2.10–1.89(m,4H,8-H, 11-H),1.93–1.80(m,2H,43-H),1.50–1.25(m,22H),0.89(q,J=7.6,6.9Hz,3H).
[0046] MS(ESI) m / z: 663.45[2M+H] +
[0047] The chemical structural formula is as follows: C 18 h 35 Br.
Embodiment 2
[0048] Embodiment 2: the preparation of gambogic acid-oleyl alcohol
[0049]
[0050] Add 1.03g gambogic acid to the reaction vessel, add N,N-dimethylformamide to dissolve, add 598.9mg oleyl alcohol bromide, then add potassium carbonate 113.53mg, stir at room temperature, overnight, silica gel column chromatography, that is Gambogic acid compound shown in formula (I), namely gambogic acid-oleyl alcohol is obtained.
[0051] The 1HNMR and MS data of this substance are as follows:
[0052] 1HNMR (CDCl3, 300MHz) δ: 12.84(s, 1H, 6-OH), 7.53(d, J=6.9Hz, 1H, 10-H), 6.66(d, J=10.2Hz, 1H, 4-H) ,6.05–5.93(m,1H,27-H),5.46(d,1H,3-H),5.355(m,2H,50-H,51-H),5.11–4.98(m,2H,32- H,37-H),3.90–3.71(m,2H,42-H),3.47(dd,J=6.9,4.4Hz,1H,11-H),3.31(dd,J=14.6,8.1Hz,1H ,31-H),3.21–3.10(m,1H,31-H),3.08–2.89(m,2H,26-H),2.51(d,J=9.3Hz,1H,22-H),2.30( dd,J=13.4,4.8Hz,1H,21-H),2.01(q,J=6.9Hz,6H,36-H,49-H,52-H),1.88–1.35(m,28H),1.35 –1.21(m,24H),0.92–0.82(m,3H).
[0053] MS (ESI) m / z (%): 879.58 [M+H]...
Embodiment 3
[0055] Embodiment 3: Preparation of gambogic acid-oleyl alcohol / vitamin E polyethylene glycol succinate nanoparticles (GA-OA / TPGS)
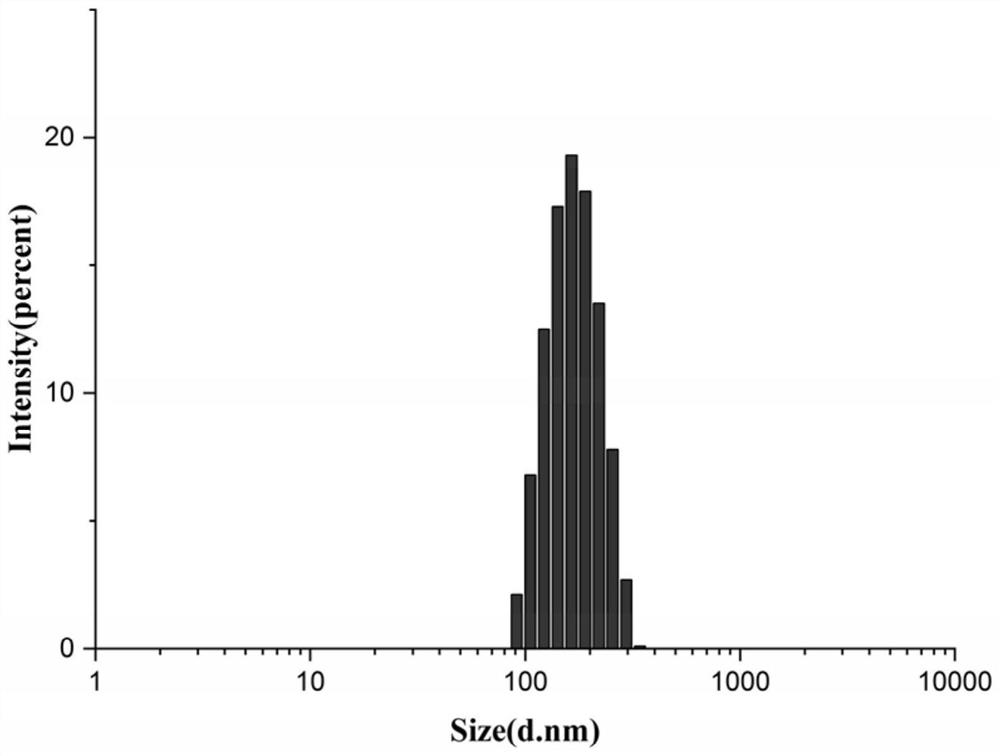
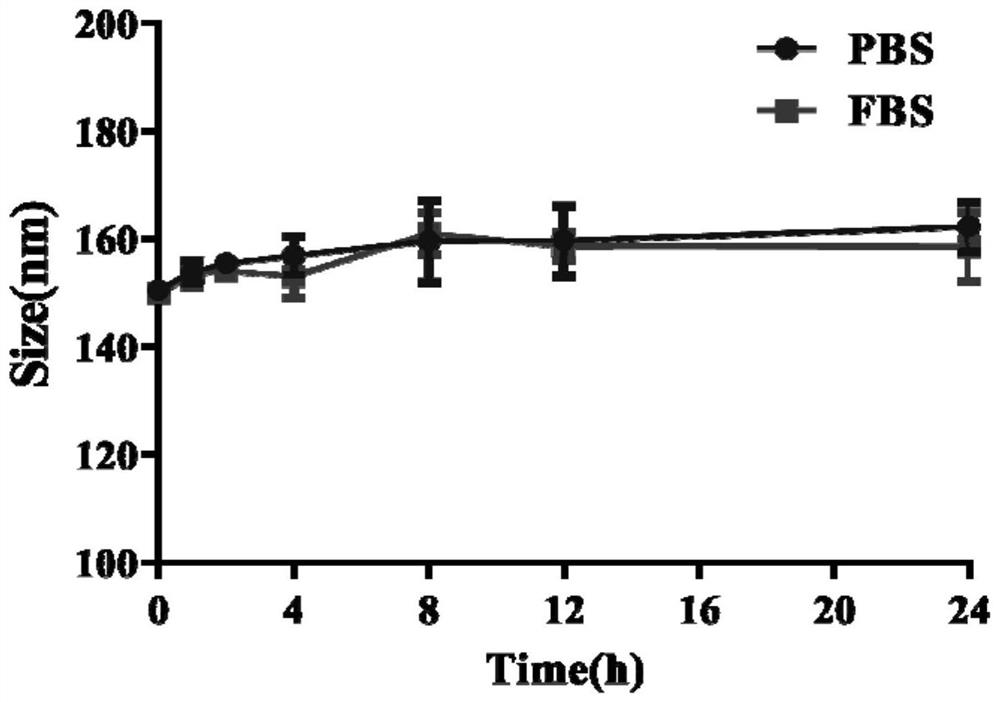
[0056] Weigh 7.15 mg of the gambogic acid-oleyl alcohol prodrug prepared in Example 2 and dissolve 1.0725 mg of vitamin E polyethylene glycol succinate (the molecular weight range of polyethylene glycol is 1000 to 10000) in 800 μl of DMSO. Slowly add the DMSO mixed solution to 10ml of ultrapure water at 37°C, stir while adding, and after standing for 2 hours, dialyze for 2 to 5 days to remove DMSO to obtain gambogic acid-oleyl alcohol / vitamin E polyethylene glycol Succinate nanoparticles (GA-OA / TPGS), the appearance of figure 1 As shown, the particle size distribution diagram is shown in figure 2 As shown, the average particle size is 155.23±3.34nm, the particle size distribution is narrow, the PDI is 0.078±0.027, and the drug loading and encapsulation efficiency are high, which are 56.82% and 65.60%, respectively. Gambogic acid-oleyl alcohol / ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com