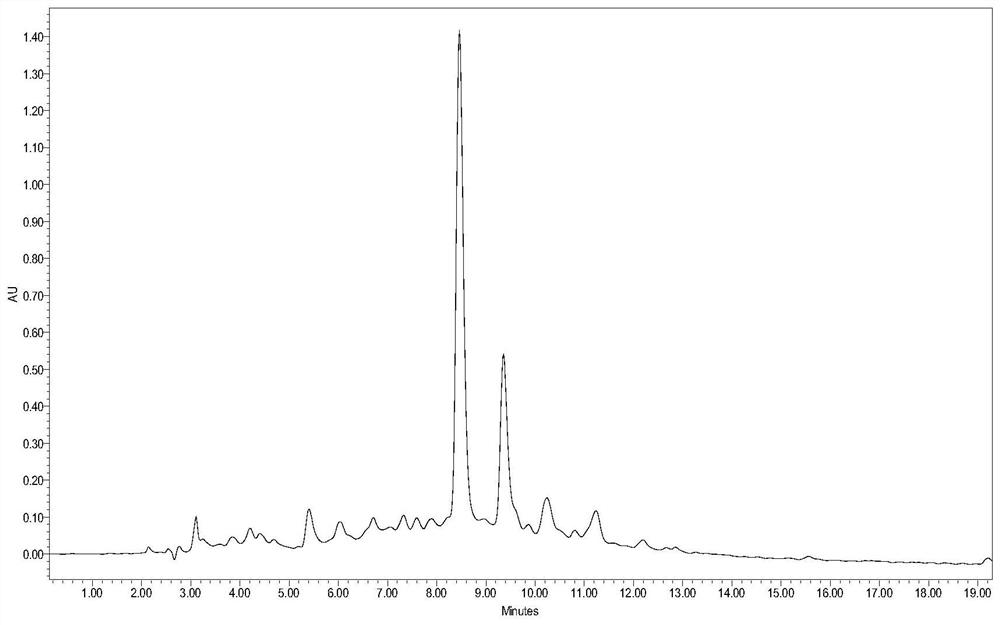
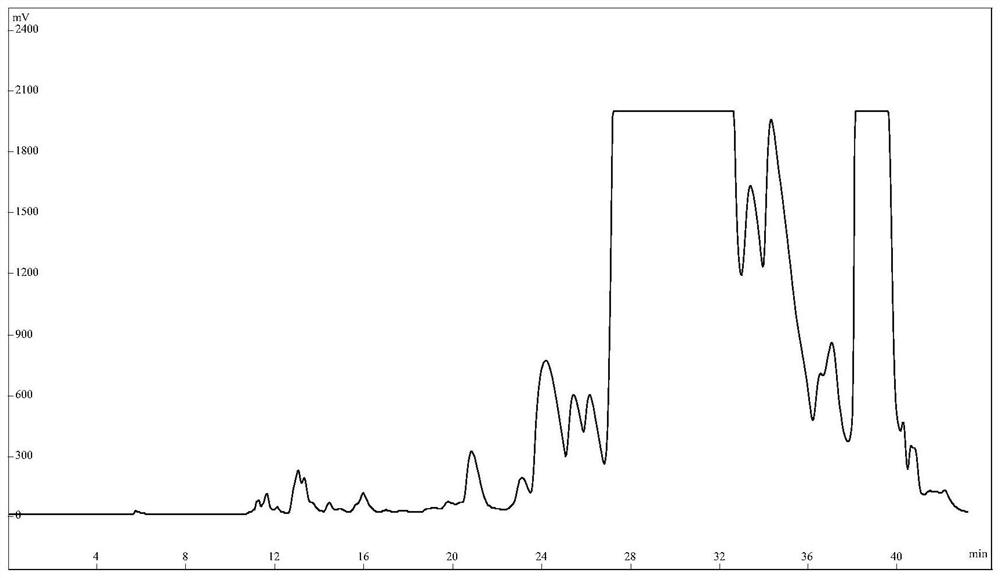
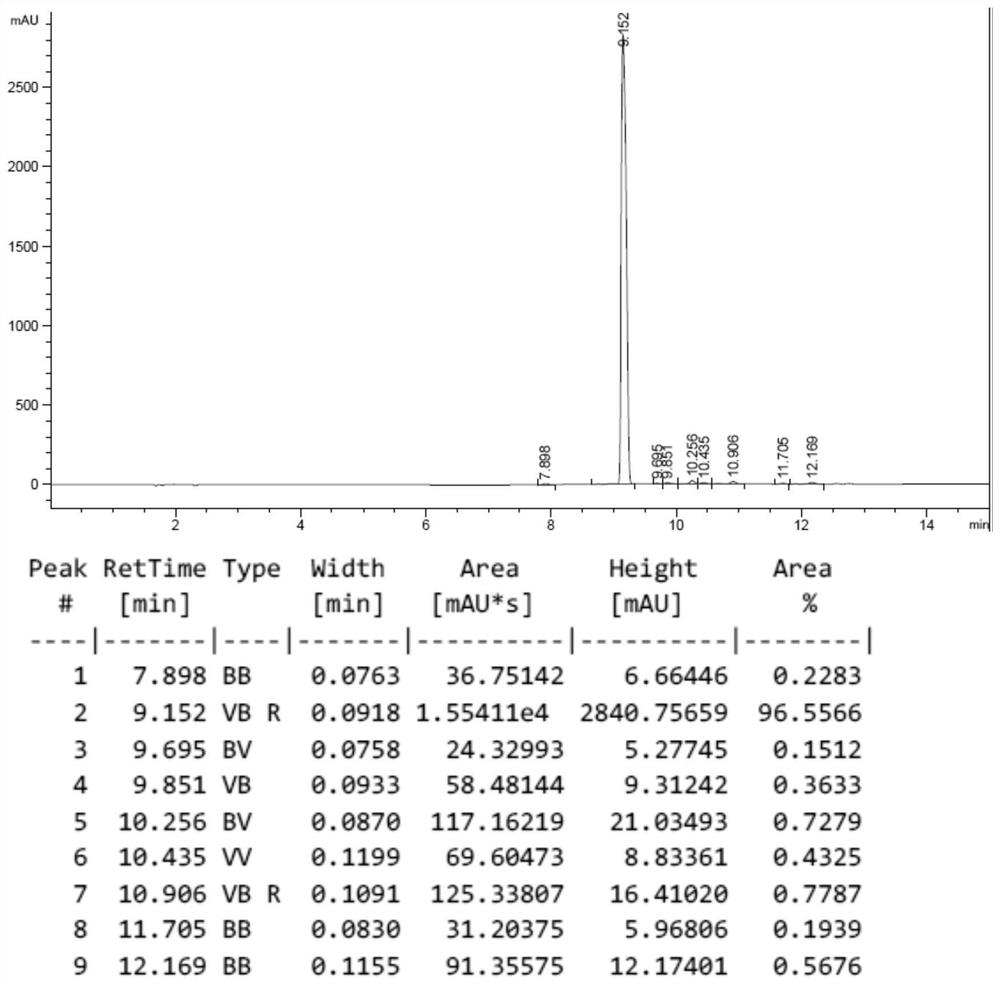
Purification method of semaglutide
A purification method and crude peptide technology, which is applied in the field of separation and purification of semaglutide, can solve the problems of unstable dissolution method of crude peptide samples, unclear removal of specific impurities, and low purification yield, so as to increase the solubility, Avoid clogging with equipment and improve the separation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1 Crude Peptide Pretreatment
[0049] Dissolution buffer preparation: Measure 2 mL of phosphoric acid and pour it into 750 mL of water, adjust the pH to 8.0 with triethylamine, add 200 mL of acetonitrile, and dilute to 1 L with water. Take 1 L of the prepared dissolution buffer, add 30 g of crude peptide, stir ultrasonically to completely dissolve the sample, filter it with a 0.22 μm organic filter membrane, and collect the filtrate.
Embodiment 2
[0050] Embodiment 2 first step purification
[0051] Purification conditions: chromatographic column: a chromatographic column with octaalkylsilane bonded silica gel as the stationary phase, and the diameter and length of the column are: 5cm×25cm. Mobile phase: Phase A: aqueous solution containing 0.2% phosphoric acid (v / v) and 20% acetonitrile (v / v), adjusted to pH 8.0 with triethylamine; phase B: acetonitrile, flow rate: 80mL / min, gradient: B% : 20%-40%, linear gradient elution 60min; detection wavelength: 230nm. The injection volume was 5.0 g. Components with a purity greater than 95% and less than 0.8% are collected for rotary distillation.
[0052] Among them, the preparation method of phase A is to measure 2 mL of phosphoric acid and pour it into 750 mL of water, adjust the pH to 8.0 with triethylamine, add 200 mL of acetonitrile, and dilute to 1 L with water.
Embodiment 3
[0053] Embodiment 3 first step purification fraction concentration
[0054] The collected semaglut first-step purified fractions were mixed and concentrated by rotary evaporation under reduced pressure at a water temperature of 30°C and a vacuum of -0.09Mbar to remove most of the organic solvents before use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com