Penetration enhancer for nasal delivery and application thereof
A nasal cavity and permeation enhancer technology, applied in non-active ingredient medical preparations, medical preparations containing active ingredients, drug combinations, etc., can solve the irreversible physiological damage of the nasal cavity, increase the solubility of insoluble drugs, and weak transmembrane ability And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
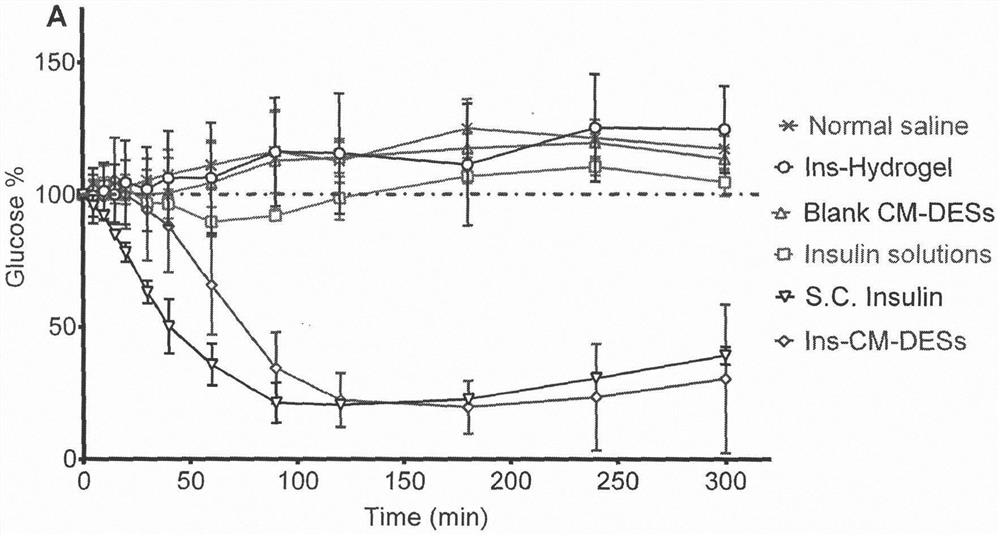
[0029] Example 1 Choline Malate System Promotes Insulin Nasal Absorption
[0030] Insulin is a biomacromolecular drug that has been widely used as a specific drug for the treatment of diabetic patients. Due to insulin's large molecular weight, strong hydrophilicity, and weak transmembrane transport ability, only injections of insulin are effective at present, and the clinical compliance is very poor. At present, many studies have attempted to make insulin into other dosage forms, such as oral, nasal, transdermal and other routes of administration. Compared with oral administration, the environment of the nasal cavity is relatively mild, the enzyme activity is low, and protein drugs are not easy to degrade; while compared with the skin, the barrier effect of the nasal mucosa is weak, and there are a large number of capillaries and lymphatic vessels under the epithelial cells, and the drug can be rapidly released. absorb. Therefore, nasal administration has been considered as ...
Embodiment 2
[0036] Example 2 Aqueous Malate Choline System Promotes Insulin Nasal Absorption
[0037] The choline malate system containing insulin was prepared, mixed with a certain amount of water, and used in the nasal administration hypoglycemic experiment. See Table 2 for detailed component descriptions.
[0038] Table 2. The mass percentages of the detailed components of the insulin-choline malate system (moisture content=40%)
[0039] DL-malic acid (Sinopharm Group) Choline Chloride (Sinopharm Group) Porcine insulin (Xuzhou Wanbang) distilled water 20% 40% 1% 40%
[0040] Method: Add malic acid and choline chloride into a beaker at a weight ratio of 1:2 and mix evenly. Heat the mixture to 80°C and keep stirring for about 3 hours until the solid disappears completely. After cooling to room temperature, add 1% insulin Add to the system, add 40% distilled water, and mix well.
[0041] In this embodiment, choline malate was used to dissolve insulin, mixed wi...
Embodiment 3
[0042] Embodiment 3 choline citrate system is used to promote propranolol nasal cavity absorption
[0043] The choline citrate system containing propranolol was prepared for nasal absorption experiment, and the detailed components are shown in Table 3.
[0044] Table 3. Mass percentages of detailed components of the propranolol-choline citrate system (moisture content=0)
[0045] Citric Acid Monohydrate (Sinopharm Group) Choline bicarbonate (Sigma) Propranolol (Sinopharm Group) 29.5% 69.5% 1%
[0046] Method: Dissolve citric acid and choline bicarbonate in methanol at a molar ratio of 1:3, stir at room temperature for 4 hours until carbon dioxide generation stops, remove methanol and water by rotary evaporation at 60°C, and vacuum dry for 48 hours to remove residual solvent. To enhance penetration system. Add 1% propranolol into the system, stir and dissolve.
[0047] The present embodiment takes rats as the animal model, administers propranolol-chol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com