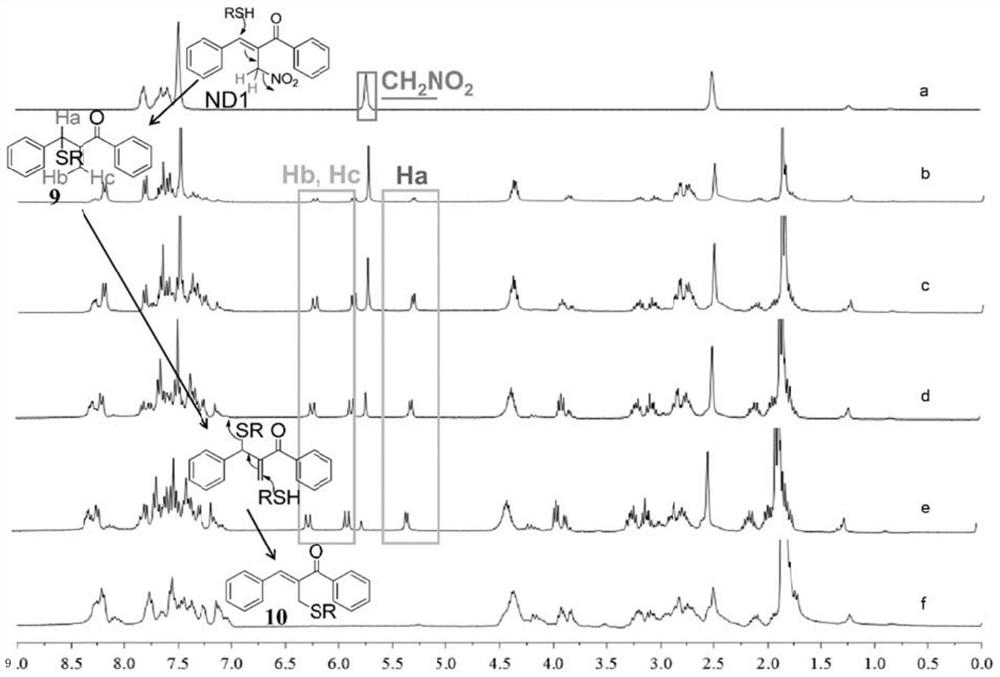
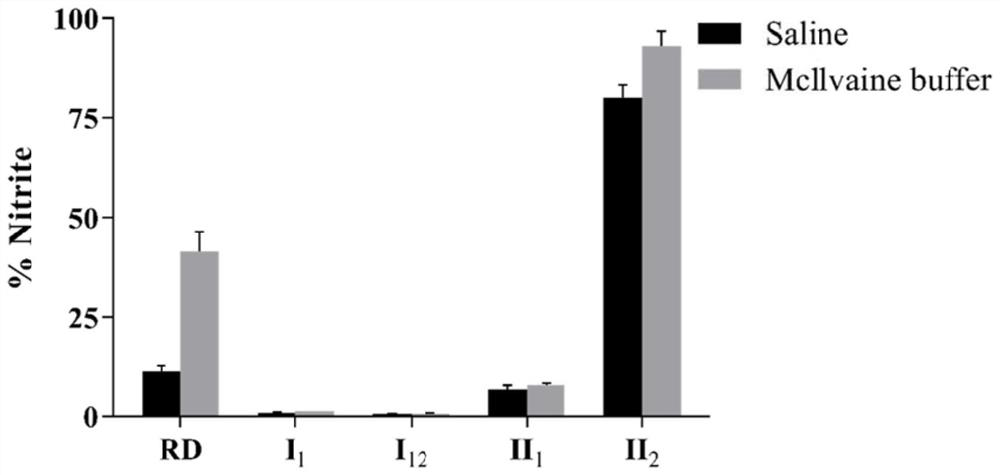
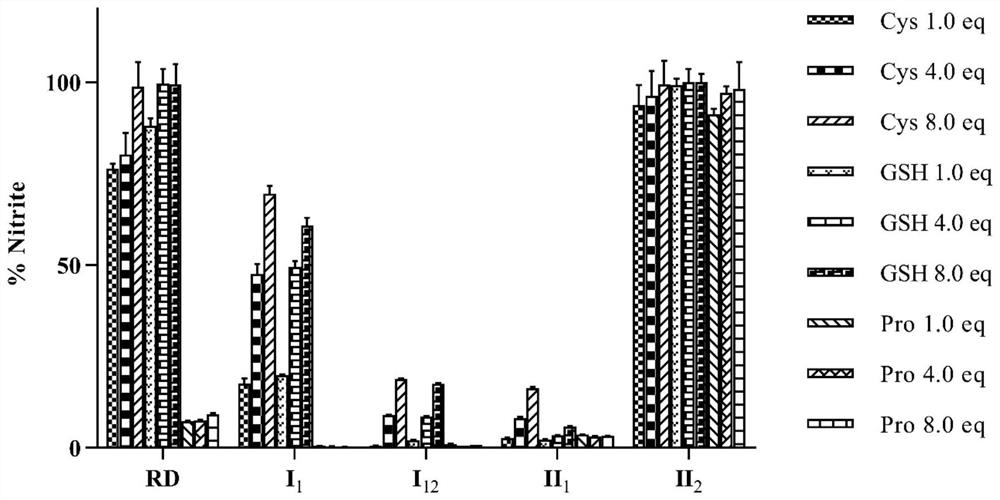
Organic nitrite donor as well as preparation method and medical application thereof
A kind of nitrite, donor technology, applied in organic nitrite donor compound and its preparation, application in myocardial ischemia and pulmonary arterial hypertension medicine, preparation prevention or treatment of cerebral ischemia field, can solve unreported biological activity It can reduce the volume of cerebral infarction and cerebral water content, reduce the volume of cardiac ischemia, and improve hemodynamics.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Embodiment 1: (E)-2-nitromethyl-1,3-diphenyl-2-en-1-one (I 1 ) preparation
[0072]
[0073] (a) Dissolve propiophenone (1.34g, 10.0mmol, 1.0eq) in 20mL of absolute ethanol, add benzaldehyde (1.17g, 11.0mmol, 1.1eq), NaOH (1.2g, 30.0mmol, 3.0eq), React overnight at 70°C. The reaction solution was acidified to pH 2-3 with dilute hydrochloric acid, then extracted with ethyl acetate (30 mL), the organic layer was washed three times with water and saturated brine, dried over anhydrous sodium sulfate, concentrated, and column chromatography (petroleum ether / ethyl acetate Ester=100 / 1, v / v) to obtain colorless oil V 1 , yield 65%. ESI-MS(m / z):223.1[M+H] + ; 1 H NMR (300MHz, CDCl 3 ): δ7.72–7.78(m,2H),7.51–7.58(m,1H),7.31–7.50(m,7H),7.18(d,1H,J=1.4Hz),2.28(d,3H,J = 1.4Hz); 13 C NMR (75MHz, CDCl 3 ): δ199.21, 142.08, 138.43, 136.72, 135.65, 131.57, 129.68, 129.33, 128.59, 128.31, 128.16, 14.37.
[0074] (b) put V 1 (222.1mg, 1.0mmol, 1.0eq) was dissolved in 15mL of...
Embodiment 2
[0076] Example 2: (E)-3-(2,5-dimethoxyphenyl)-2-nitromethyl-1-phenylprop-2-en-1-one (I 2 ) preparation
[0077]
[0078] Referring to the synthetic method of Example 1. ESI-MS(m / z):328.1[M+H] + ; 1 H NMR (300MHz, MeOD) δ7.75(d, J=7.9Hz, 2H), 7.58(t, J=7.2Hz, 1H), 7.46(s, 2H), 7.31(s, 1H), 7.09(s ,1H),6.98–6.73(m,2H),5.59(s,2H),3.75(s,3H),3.68(s,3H). 13 C NMR (75MHz,MeOD)δ197.50,153.80,150.78,140.04,137.23,132.39,129.15,127.96,122.87,115.97,115.46,112.99,112.73,111.39,66.63,54.838,54.7
Embodiment 3
[0079] Embodiment 3: (E)-3-(4-methoxyphenyl)-2-nitromethyl-1-phenylprop-2-en-1-ketone (I 3 ) preparation
[0080]
[0081] Referring to the synthetic method of Example 1. ESI-MS(m / z):298.1[M+H] + ; 1 H NMR (300MHz, CDCl 3 )δ7.83(d, J=8.4Hz, 2H), 7.58(s, 1H), 7.51(t, J=7.3Hz, 2H), 7.35–7.21(m, 3H), 6.98(d, J=8.8 Hz,2H),5.64(s,2H),3.73(s,3H). 13 C NMR (75MHz, CDCl 3 )δ196.61, 149.04, 144.00, 132.95, 131.23, 129.67, 128.49, 125.82, 124.02, 118.30, 114.71, 72.24, 58.34.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com