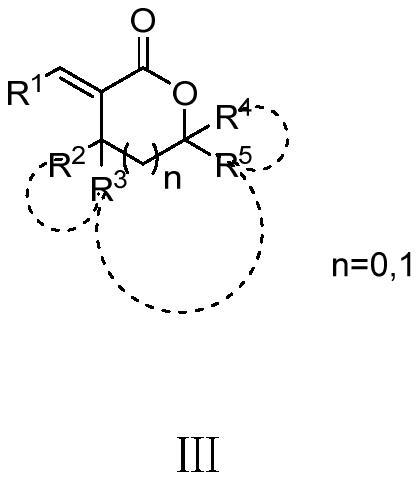
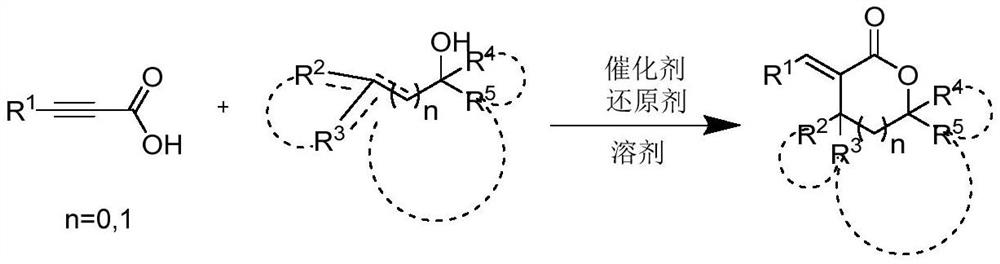
Polysubstituted alpha-alkenyl lactone compound and preparation method and application thereof
An alkenyl lactone, multi-substituted technology, applied in the directions of organic chemistry, drug combination, anti-infective drugs, etc., can solve the problems of many reaction steps, low yield, etc., and achieves low cost, good yield, and good application prospects. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Target compound:
[0039]
[0040] Preparation method: add 3.0g of phenylpropylic acid and 2.3g of ferric oxalate to the reaction bottle, add 7ml of acetonitrile and methanol mixed solvent, weigh 1.5g of 2-methallyl alcohol, 2.6g of triethylsilane, and react at room temperature After 8 hours, it was quenched by adding water, extracted with ethyl acetate, and recrystallized to obtain 3.4 g of white solid, with a yield of 82%.
[0041] Product characterization: white solid, melting point, 96-97°C, 1 H NMR (400MHz, CDCl3 ):δ7.67 (s,1H),7.43-7.32(m,5H),3.96(s,2H),1.28(s,6H). 13 C NMR (101MHz, CDCl 3 ): δ172.54, 138.14, 135.27, 134.18, 129.27, 128.86, 128.26, 78.96, 39.48, 25.70. HRMS (m / z): calcd for C 13 h 14 NaO 2 + [M + Na] + :225.0886,found: 225.0892.
Embodiment 2
[0043] Target compound:
[0044]
[0045] Preparation:
[0046] Preparation method: Add 3.6g of p-chlorobenzoic acid, 2.6g of nickel acetylacetonate in sequence, add 5ml of mixed solvent of ethanol and THF, weigh 2.8g of cyclohex-1-en-1-ylmethanol, 4.0g of benzene Silane was refluxed for 3 hours, quenched by adding water, extracted with ethyl acetate, and separated by column chromatography to obtain 4.1 g of white solid with a yield of 86%.
[0047] Product characterization: white solid, melting point, 124-125°C. 1 H NMR (400MHz, CDCl 3 ):δ 7.73(s,1H),7.47-7.33(m,5H),4.18(s,2H),1.82-1.75(m,2H),1.72-1.60(m, 5H),1.30-1.17(m, 2H),1.18-1.04(m,1H). 13 C NMR (101MHz, CDCl 3 ): δ 172.88, 138.66, 135.23, 134.40, 129.25, 128.87, 128.61, 128.22, 74.76, 43.61, 33.63, 24.90, 22.48. HRMS (m / z): calcd for C 16 h 18 o 2 [MH] + :243.13763,Found: 243.13763.
Embodiment 3
[0049] Target compound:
[0050]
[0051] Preparation:
[0052] R in the reactant 1 = phenyl, R 2 +R 3 = Cyclopentyl, R 4 , R 5 = hydrogen, n = 0; solvent is toluene, catalyst is FeCl 3 , the reducing agent is HSiCl 3 ; The molar ratio of reactants I and II is 1:0.3; the molar ratio of reactant I and catalyst is 1:0.05; the molar ratio of reactant I and reducing agent is 1:0.5. The reactant, solvent, catalyst and reducing agent were added into the reaction flask in sequence, refluxed for 2 hours, quenched with water, extracted with ethyl acetate, and separated by column chromatography to obtain a white solid with a yield of 80.2%.
[0053] Product characterization: white solid, melting point, 78-79°C. 1 H NMR (400MHz, CDCl 3 ):δ7.65 (s,1H),7.42-7.31(m,5H),4.00(s,2H),2.09-2.01(m,2H),1.82-1.72(m,2H), 1.70-1.59(m ,4H). 13 C NMR (101MHz, CDCl 3 ): δ173.02, 137.48, 134.13, 132.93, 129.58, 129.01, 128.82, 128.52, 128.30, 79.82, 50.19, 36.55, 25.33. HRMS (m / z): calcd f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com