Synthesis method of 2-phenylacetylene seleno alcohol compound
A synthetic method and technology of phenylacetylene, applied in the direction of organic chemistry, can solve the problems of poor functional group tolerance, pre-preparation, narrow substrate range, etc., and achieve the effects of simple post-processing, high yield and purity, and high reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
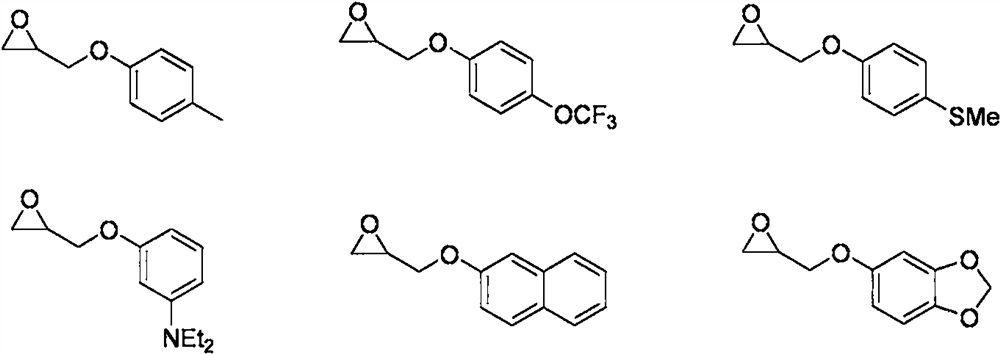
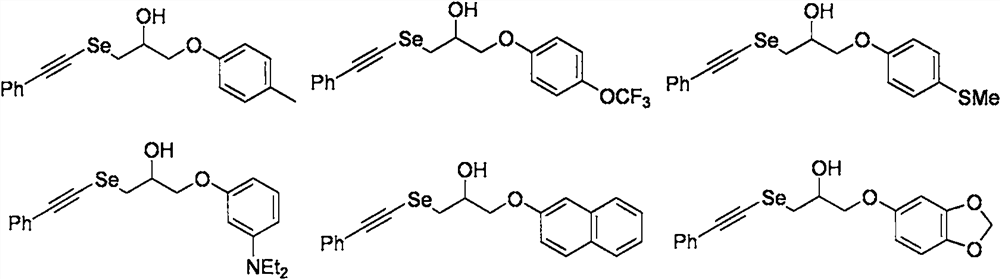
[0031] Synthesis of 1-phenylacetylene selenyl-3-(4-methylphenoxy)-2-propanol compound
[0032]
[0033] At room temperature, phenylpropiolic acid (0.2mmol), elemental selenium (0.6mmol), 2-(4-methylphenoxy)methyloxirane (0.6mmol), copper chloride (0.02mmol) , 1,10-phenanthroline (0.02mmol), cesium carbonate (0.6mmol), tetrabutylammonium iodide (0.4mmol) and 2mL water, stirred at a reaction temperature of 50°C for 24h. After the reaction is over, add ethyl acetate to dilute, transfer the diluted solution to a separatory funnel for extraction, separate the aqueous phase and the organic phase, then extract the aqueous phase with ethyl acetate for 3 times, combine the organic phases, and add 5g of anhydrous sulfuric acid Sodium, stand still for 30min, wash the filter cake 3 times with 5mL ethyl acetate each time, then spin off the solvent, and obtain the product through column chromatography (eluent: sherwood oil: ethyl acetate=20: 1), the product is Pale yellow liquid, yield ...
Embodiment 2
[0041] Synthesis of 1-phenylacetylene selenyl-3-(4-trifluoromethoxyphenoxy)-2-propanol compound
[0042]
[0043] At room temperature, phenylpropiolic acid (0.2mmol), elemental selenium (0.6mmol), 2-(4-trifluoromethoxyphenoxy)methyloxirane (0.6mmol), copper chloride ( 0.02mmol), 1,10-phenanthroline (0.02mmol), cesium carbonate (0.6mmol), tetrabutylammonium iodide (0.4mmol) and 2mL water, stirred at a reaction temperature of 50°C for 24h. After the reaction is over, add ethyl acetate to dilute, transfer the diluted solution to a separatory funnel for extraction, separate the aqueous phase and the organic phase, then extract the aqueous phase with ethyl acetate for 3 times, combine the organic phases, and add 5g of anhydrous sulfuric acid Sodium, stand still for 30min, wash the filter cake 3 times with 5mL ethyl acetate each time, then spin off the solvent, and obtain the product through column chromatography (eluent: sherwood oil: ethyl acetate=20: 1), the product is Light ...
Embodiment 3
[0053] Synthesis of 1-phenylacetylene selenyl-3-(4-thiomethylphenoxy)-2-propanol compound
[0054]
[0055] At room temperature, phenylpropiolic acid (0.2mmol), elemental selenium (0.6mmol), 2-(4-thiomethylphenoxy)methyloxirane (0.6mmol), copper chloride (0.02mmol ), 1,10-phenanthroline (0.02mmol), cesium carbonate (0.6mmol), tetrabutylammonium iodide (0.4mmol) and 2mL water, stirred at a reaction temperature of 50°C for 24h. After the reaction is over, add ethyl acetate to dilute, transfer the diluted solution to a separatory funnel for extraction, separate the aqueous phase and the organic phase, then extract the aqueous phase with ethyl acetate for 3 times, combine the organic phases, and add 5g of anhydrous sulfuric acid Sodium, stand still for 30min, wash the filter cake 3 times with 5mL ethyl acetate each time, then spin off the solvent, and obtain the product through column chromatography (eluent: sherwood oil: ethyl acetate=20: 1), the product is Light yellow solid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com