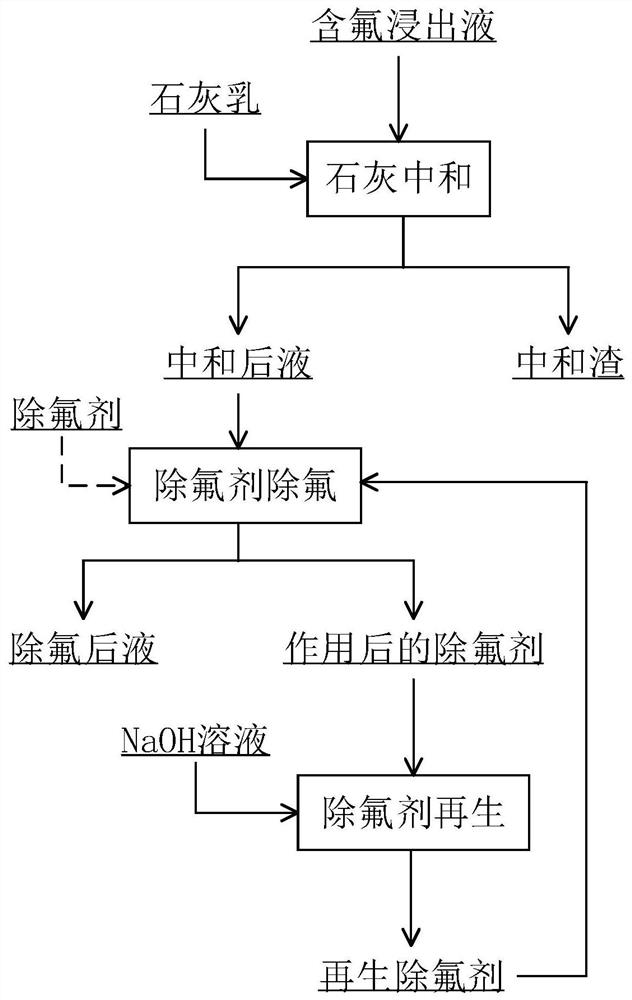
Two-stage fluorine removal method for fluorine-containing material
A technology for materials and fluorine removal agents, which is applied in the fields of hydrometallurgy and resource recovery, can solve the problems of not being suitable for handling solutions with high fluorine content, long time for fluorine removal, and no method for efficient fluorine removal has been reported. The effect of high fluorine efficiency, avoiding large dosage and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031]The fluorine-containing solid powder used in this embodiment is waste battery powder, and its main components are shown in Table 1:
[0032]Table 1 Main element content of raw materials (%)
[0033] element Co Ni Mn F content 16.0937.4116.581.40
[0034]1. Mix water and raw materials into a slurry according to the liquid-to-solid ratio of 3:1~10:1, and react for 0.5~3.5h under the conditions of 60~90℃ and stirring speed of 250rpm~400rpm. Add 10~18.4mol / during the reaction. L sulfuric acid adjusts the pH to control the end point pH of the reaction to be 0.5-2.5. After the reaction is over, the leachate and filter residue are obtained by vacuum suction filtration, and then the content of related elements in the leachate is measured. The content of the main elements is shown in Table 2:
[0035]Table 2 Main element content of leaching solution (g / L)
[0036] element Co Ni Mn F content 15.4631.8124.201.50
[0037]2. Take a certain volume of the leaching solution, add a calcium carbonate solu...
Embodiment 2
[0047]The fluorine-containing solid powder used in this embodiment is waste battery powder, and its main components are shown in Table 5:
[0048]Table 5 Main element content of raw materials (%)
[0049] element Co Ni Mn F content 16.0937.4116.581.40
[0050]1. Mix water and raw materials into a slurry according to the liquid-to-solid ratio of 3:1~10:1, and react for 0.5~3.5h under the conditions of 60~90℃ and stirring speed of 250rpm~400rpm. Add 10~18.4mol / during the reaction. L sulfuric acid adjusts the pH to control the end point pH of the reaction to be 0.5-2.5. After the reaction is over, the leachate and filter residue are obtained by vacuum filtration, and then the content of related elements in the leachate is measured. The content of the main elements is shown in Table 6:
[0051]Table 6 Main element content of leaching solution (g / L)
[0052] element Co Ni Mn F content 15.4631.8124.201.50
[0053]2. Take a certain volume of leaching solution, add 10%-30% calcium hydroxide solution a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com