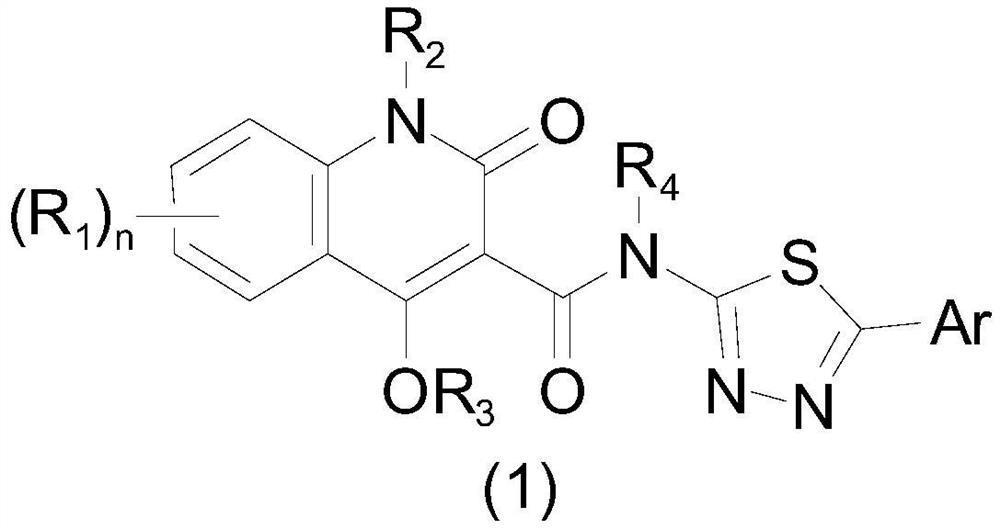
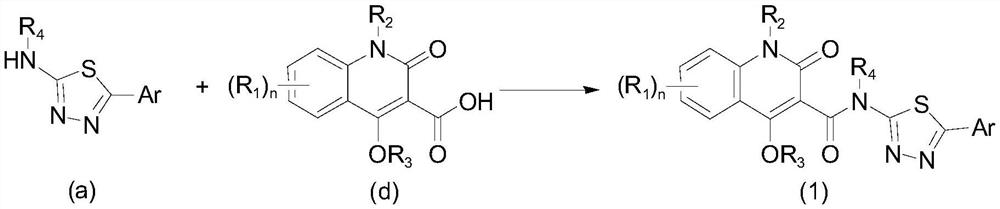
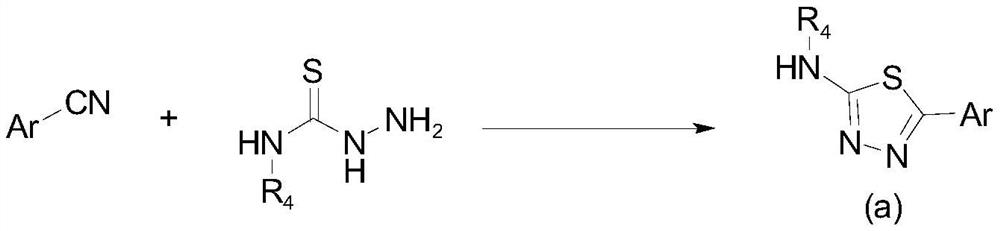
Aryl or heteroaryl substituted thiadiazole compound and antibacterial application thereof
A compound, heteroaryl technology, applied in the field of antibacterial drugs, can solve serious problems such as the slowdown of antibacterial drug research and development, and antibiotic resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] Embodiment 1: Preparation and detection of compound J1
[0096]
[0097] Add benzonitrile (1.03g, 10mmol) to a 100mL eggplant-shaped bottle, dissolve it with 10mL trifluoroacetic acid (TFA) and stir for 5 minutes, then add thiosemicarbazide (1.00g, 11mmol) in batches, and then add trifluoroacetic acid (TFA) 10 mL of acetic acid, and the mixed solution was reacted at 70° C. for 8 hours. The reaction was monitored by thin-layer chromatography, and when the benzonitrile was consumed, the heating was stopped and allowed to cool naturally. Then, the reaction solution was poured into ice water, and the pH was adjusted to 11-12 with 1 normality of sodium hydroxide solution, and a large amount of yellow precipitates could be seen. Suction filtration, the filter cake was washed with water (150mL) and suction filtration to obtain the crude product. Recrystallization was performed with 95% ethanol to obtain 1.21 g of the target product (yield: 67.9%).
[0098] 12.8 mL (73.6 ...
Embodiment 2
[0104] Embodiment 2: Preparation and detection of compound J2
[0105] Taking 4-chlorobenzonitrile as the starting raw material, the synthesis method is the same as the preparation of the intermediate 5-phenyl-1,3,4-thiadiazol-2-amine mentioned in Example 1 to obtain the intermediate 5-( 4-Chlorophenyl)-1,3,4-thiadiazol-2-amine (yield: 77.2%).
[0106] Using 5-(4-chlorophenyl)-1,3,4-thiadiazol-2-amine and 1-ethyl-4-hydroxyl-2-quinolone-3-carboxylic acid as starting materials, the synthesis method is the same as the implementation The preparation of the final product J1 mentioned in Example 1 gave N-(5-(4-chlorophenyl)-1,3,4-thiadiazol-2-yl)-1-ethyl-4-hydroxy- 2-Quinolone-3-carboxamide (J2).
[0107]
[0108] Pale yellow solid, yield 31.5%. ESI-MS(m / z):428.12[M+H] + . 1 H NMR (500MHz, DMSO-d 6)δ7.80(d, J=8.2Hz, 2H), 7.55(d, J=8.3Hz, 3H), 7.50(s, 3H), 4.27(dd, J=19.5, 12.3Hz, 2H), 1.22( t,J=7.1Hz,3H).
Embodiment 3
[0109] Embodiment 3: Preparation and detection of compound J3
[0110] Taking 4-bromoxynil as the starting material, the synthesis method is the same as the preparation of the intermediate 5-phenyl-1,3,4-thiadiazol-2-amine mentioned in Example 1 to obtain the intermediate 5-( 4-bromophenyl)-1,3,4-thiadiazol-2-amine (yield: 55.3%).
[0111] Using 5-(4-bromophenyl)-1,3,4-thiadiazol-2-amine and 1-ethyl-4-hydroxyl-2-quinolone-3-carboxylic acid as starting materials, the synthesis method is the same as the implementation The preparation of the final product J1 mentioned in Example 1 gave N-(5-(4-bromophenyl)-1,3,4-thiadiazol-2-yl)-1-ethyl-4-hydroxy- 2-Quinolone-3-carboxamide (J3).
[0112]
[0113] Off-white solid, yield 19.2%. ESI-MS(m / z):472.31[M+H] + . 1 H NMR (500MHz, DMSO-d 6 )δ7.73(d, J=8.5Hz, 2H), 7.69(d, J=8.4Hz, 3H), 7.51(s, 3H), 4.30(p, J=6.8Hz, 2H), 1.23(t, J=7.1Hz,3H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com