Preparation method of self-supporting structure zinc-copper alloy negative electrode
A self-supporting structure, zinc-copper alloy technology, applied in electrode manufacturing, structural parts, battery electrodes, etc., can solve the problems of complex operation of electrochemical deposition methods, difficulty in ensuring alloy uniformity, and difficulty in mass production, etc., to improve the cycle. Reversibility, reduction of zinc dendrite formation, effect of improving lifetime and performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] S1. Surface pretreatment of zinc block and copper mesh: prepare zinc block, copper mesh, alcohol, concentrated hydrochloric acid, acetone; use wire brush, sandpaper or grinding wheel to remove oxides on the surface of zinc block, and then wipe with acetone and alcohol to remove surface oil , set aside; wipe the copper grid with acetone and alcohol to remove surface oil, then soak it with concentrated hydrochloric acid to remove oxides on the surface of the copper grid, take out the copper grid, wipe it again with alcohol to remove residual hydrochloric acid, then dry it with a hair dryer, and set aside;
[0050] S2. Put the zinc block into the graphite crucible, heat and smelt in the electromagnetic induction furnace with argon protection atmosphere, and spontaneously stir the zinc melt under the action of electromagnetic force to ensure the uniformity of smelting;
[0051] S3. Control the temperature of the pure zinc liquid to 600°C, take out the molten zinc liquid, rem...
Embodiment 2
[0054] S1. Grind the zinc block and the copper mesh with a wire brush to remove the surface oxide, then clean them with acetone and alcohol, soak the copper mesh with concentrated hydrochloric acid, wipe it again with alcohol after taking it out, and then dry it with a hair dryer;
[0055] S2. Put the zinc block into the graphite crucible, heat and smelt in the electromagnetic induction furnace with argon protection atmosphere, and spontaneously stir the zinc melt under the action of electromagnetic force to ensure the uniformity of smelting;
[0056] S3. Control the temperature of the pure zinc liquid to 500°C, take out the molten zinc liquid, remove the slag and oxides on the surface of the solution, and put the 200-mesh H65 brass mesh with a wire diameter of 0.051 mm into the molten zinc for 8 seconds. (approximately 1t) and take it out for natural cooling to obtain the required zinc-copper alloy.
[0057] S4, making the prepared zinc-copper alloy into negative electrode di...
Embodiment 3
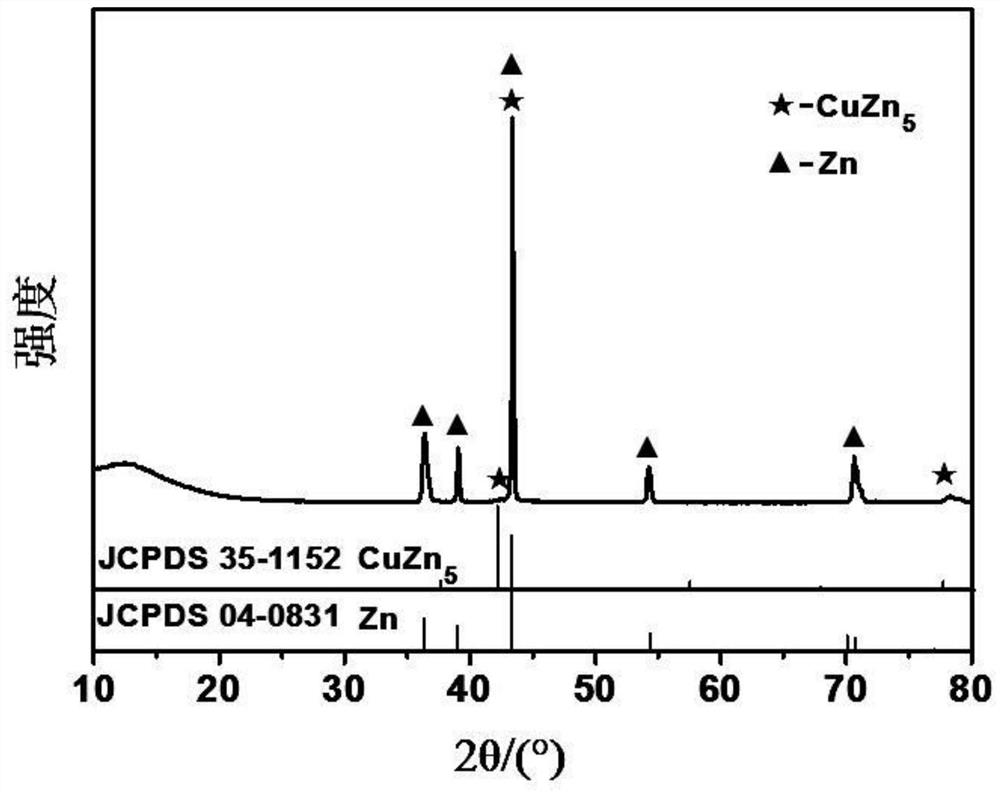
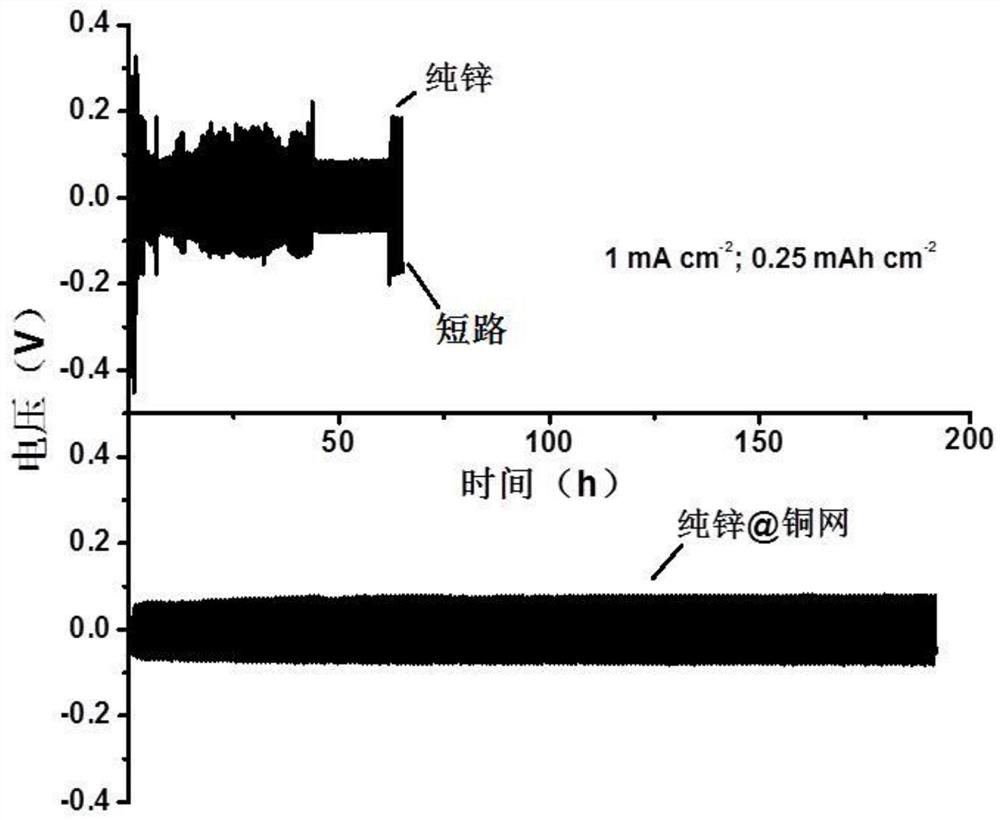
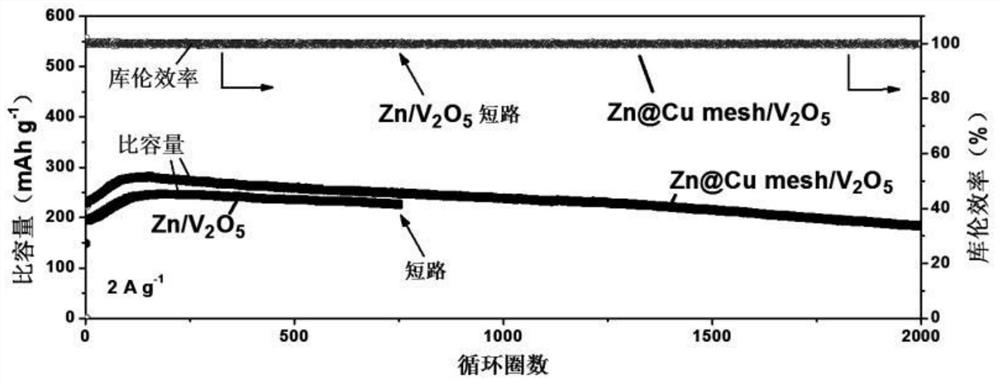
[0059] Performance evaluation (1) of the electrode that zinc-copper alloy of the present invention is made
[0060] The negative electrode pellets obtained in Example 1 are assembled into a symmetrical battery, at 1mA cm -2 Cycling performance tests were performed under current density and compared with V 2 o 5 The positive electrode is assembled into a full battery at 2A g -1 The performance test was carried out under the current density, and the electrolyte was 3MZn(CF 3 SO 3 ) 2 , the diaphragm is glass fiber filter paper.
[0061] The electrochemical test is carried out by Wuhan Landian System (CT2001A), and the test capacity of the symmetrical battery is limited to 0.25mAh cm -2 , the electrochemical window of the full battery test is 0.3-1.6V, V 2 o 5 The preparation of the positive electrode is to V 2 o 5 Mix with conductive carbon and polyvinylidene fluoride in a ratio of 7:2:1, drop into N-methyl-2-pyrrolidone solution and stir evenly, then apply it to condu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com