A kind of synthetic method of sulfonamide compound
A synthetic method and compound technology, which is applied in the preparation of sulfonamides, organic chemistry, sulfide preparation, etc., can solve the problems of many steps, unpleasant smell, low yield, etc., and achieve simplified operation steps and substrate universality Strong, wide-ranging effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
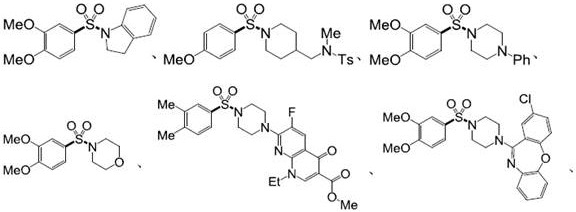
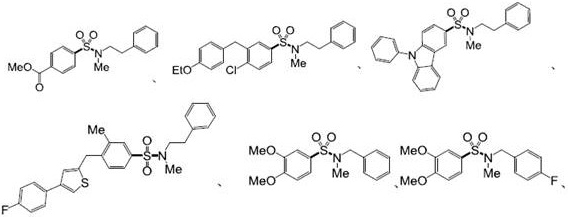
Examples
Embodiment 1
[0072] Embodiment 1. Preparation of compound 5a
[0073]
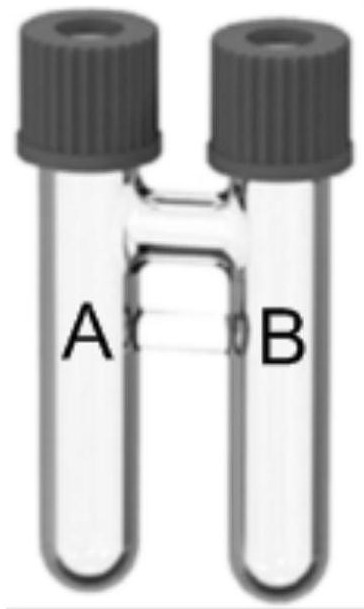
[0074] In a glove box filled with high-purity argon, DMAP (61.0mg, 2.5equiv), nBuPAD 2 (14.4mg, 20.0mol%), Pd(acac) 2 (7.6mg, 12.5mol%), 4-iodo-1,2-dimethoxybenzene 3a (0.2mmol, 1.0equiv), N-methyl-2-phenylethane-1-amine 4a (0.52mmol ,2.6equiv) add in order figure 1 In tube B equipped with a magnetic stirring bar, add 1ml DMSO at the same time. Quickly weigh compound 1 into tube A equipped with a magnetic stirrer, add 1ml of tetradecane, and then add compound 2 into tube A. Quickly tighten the bottle caps of tubes A and B, then take out the glove box, place the above device on a magnetic stirrer at 95°C and stir for 24 hours. Extract with ethyl ester and water (15ml×3 times), combine the ethyl acetate layers, dry over anhydrous sodium sulfate, concentrate, add 200-300 mesh silica gel and mix to dryness, and obtain the compound 5a shown in formula 1 through column chromatography. Yellow liquid, yield 74%.
[00...
Embodiment 2
[0076] Embodiment 2. Preparation of compound 5b
[0077] The preparation method is the same as that of compound 5a, except that 4-iodo-1,2-dimethoxybenzene is replaced by 1-ethoxy-4-iodobenzene to obtain compound 5b, a white solid, with a yield of 63%.
[0078] Compound 5b: 1 H NMR (400MHz, CDCl 3)δ7.70(d, J=8.8Hz, 2H), 7.34-7.23(m, 3H), 7.21(d, J=6.8Hz, 2H), 6.96(d, J=8.8Hz, 2H), 4.10( q,J=6.8Hz,2H),3.28-3.24(m,2H),2.90-2.86(m,2H),2.76(s,3H),1.46(t,J=6.8Hz,3H); 13 C NMR (100MHz, CDCl 3 )δ162.4, 138.5, 129.6, 129.3, 129.0, 128.7, 126.7, 114.7, 64.1, 52.0, 35.3, 35.0, 14.8; HRMS m / z calculated for C 17 h 21 NO 3 S[M+H] + :320.1315,found:320.1316.
Embodiment 3
[0079] Embodiment 3. Preparation of compound 5c
[0080] The preparation method is the same as that of compound 5a, except that 4-iodo-1,2-dimethoxybenzene is replaced with 1-iodo-4-phenoxybenzene to obtain compound 5c, a yellow liquid, with a yield of 62%.
[0081] Compound 5c: 1 H NMR (400MHz, CDCl 3 )δ7.70(d, J=8.8Hz, 2H), 7.43-7.39(m, 2H), 7.32-7.28(m, 2H), 7.23-7.18(m, 4H), 7.07(d, J=7.6Hz ,2H),7.02(d,J=8.8Hz,2H),3.29-3.25(m,2H),2.89-2.86(m,2H),2.77(s,3H); 13 C NMR (100MHz, CDCl 3 )δ161.6, 155.3, 138.4, 131.6, 130.3, 129.6, 128.9, 128.7, 126.7, 125.0, 120.4, 117.7, 51.9, 35.3, 35.0; HRMS m / z calculated for C 21 h 21 NO 3 S[M+Na] + :390.1134,found:390.1136.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com