Method for synthesizing cyclic carbonate and derivatives thereof
A technology of cyclic carbonates and synthesis methods, applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, chemical/physical processes, etc., can solve the problems of high metal residues and raw material costs, and limited metal catalysts Application, narrow applicability of catalysts, etc., to achieve good catalytic effect, low cost of raw materials, and convenient purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
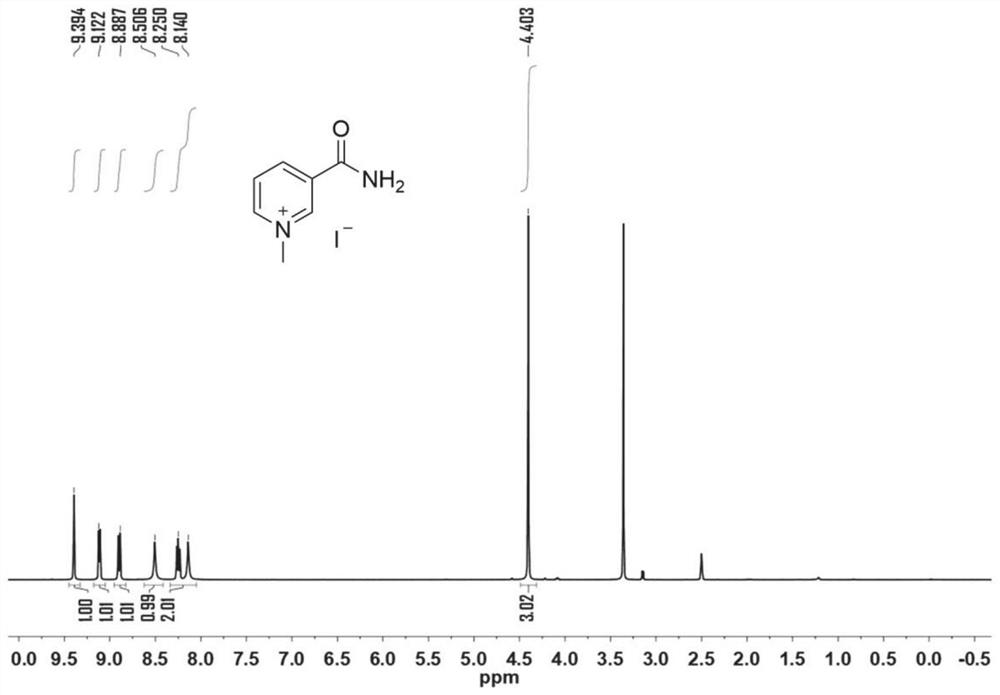
[0054] Weigh 0.244g (2mmol) of nicotinamide, add it into a 25mL pincer bottle, measure 10mL of methanol into it, shake and shake well. Then absorb 0.187mL (3mmol) of iodomethane, add it into the reaction bottle and mix well, and stir the reaction at room temperature for 2h. After the reaction was complete, a yellow solid was obtained by filtration under reduced pressure, and the obtained solid was recrystallized with 2 mL of methanol to obtain 0.32 g of a yellow crystal. That 1 HNMR spectrum such as figure 1 shown. The deuterium reagent used is DMSO-d 6 , the chemical shift is 2.5, the ratio of peak area is 1:1:1:1:2:3, which is in line with the expectation, and there is no obvious miscellaneous peak. 1 HNMR analysis can confirm that the structure of the catalyst (1) is correct.
Embodiment 2
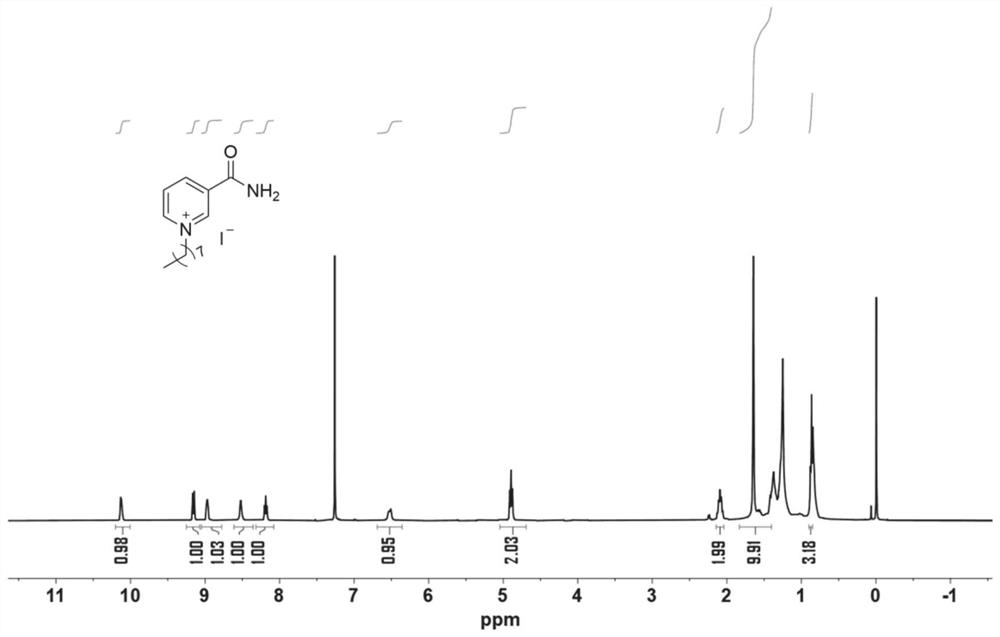
[0056] Weigh 0.488g (4mmol) of nicotinamide, add it into a 50mL pincer bottle, measure 20mL of methanol into it, shake and shake well. Then absorb 1.07 mL (6 mmol) of iodo-n-octane, add it into the reaction bottle and mix evenly, and stir the reaction at 60° C. for 12 h. After the reaction is complete, low-pressure rotary steaming to obtain a yellow oily liquid, the resulting liquid is removed by column chromatography (ethyl acetate EA as the mobile phase) to remove unreacted nicotinamide, then rinsed directly with methanol, low-pressure rotary steaming, and oven-dried at 60 ° C Overnight, 0.94 g of a white solid was obtained. That 1 HNMR spectrum such as figure 2 shown. The deuterated reagent used is CDCl 3 , the chemical shift is 7.26, the ratio of peak area is 1:1:1:1:1:1:2:2:10:3, which is in line with the expectation, and there is no obvious miscellaneous peak. 1 HNMR analysis can confirm that the structure of the catalyst (2) is correct.
Embodiment 3
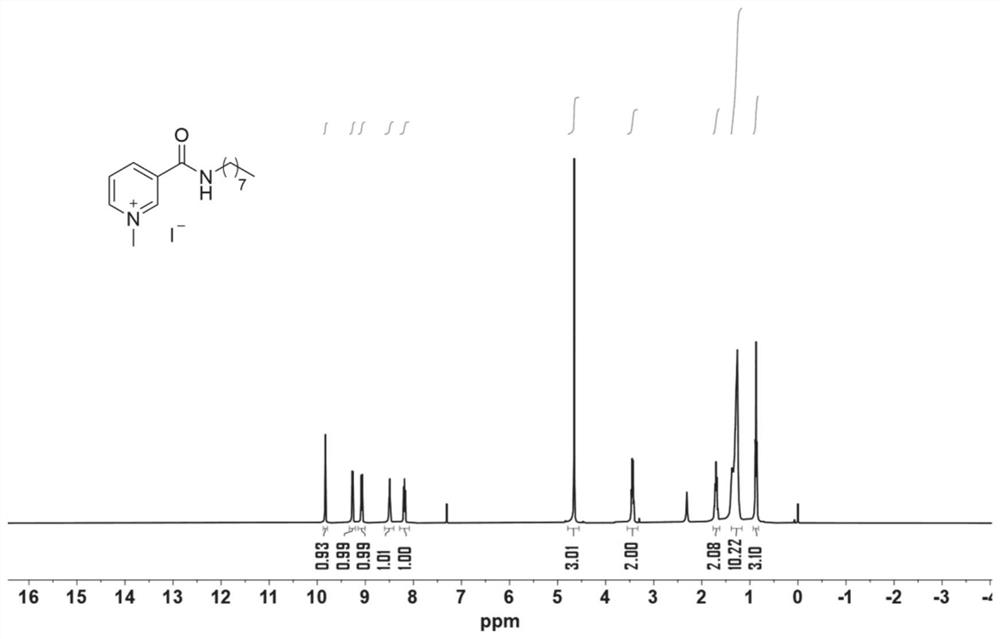
[0058] React with nicotinamide (488.4 mg, 4.0 mmol), 1-iodooctane (1.07 mL, 6.0 mmol), benzoic acid (73.2 mg, 0.6 mmol) and p-xylene (5 mL). The reaction mixture was stirred at 130°C for 8 hours. After cooling to room temperature, the resulting solution mixture was diluted with ethyl acetate (10 mL). The reaction mixture was purified by silica gel column chromatography to obtain the desired product (894.8 mg, 89%). The resulting product (502.7 mg, 2 mmol) was reacted with iodomethane (249 μL, 4.0 mmol). The reaction mixture was purified by column chromatography on silica gel to give the product as a colorless solid (322.5 mg, 41%). That 1 HNMR spectrum such as image 3 shown. The deuterated reagent used is CDCl 3 , its chemical shift is 7.26, and the ratio of peak area is 1:1:1:1:1:3:2:2:10:3, which is in line with the expectation, and there is no obvious miscellaneous peak. 1 HNMR analysis can confirm that the structure of the catalyst (3) is correct.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com