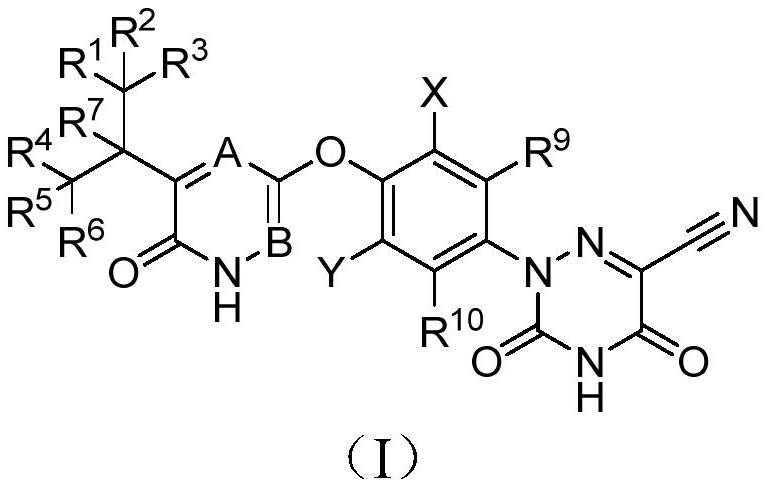
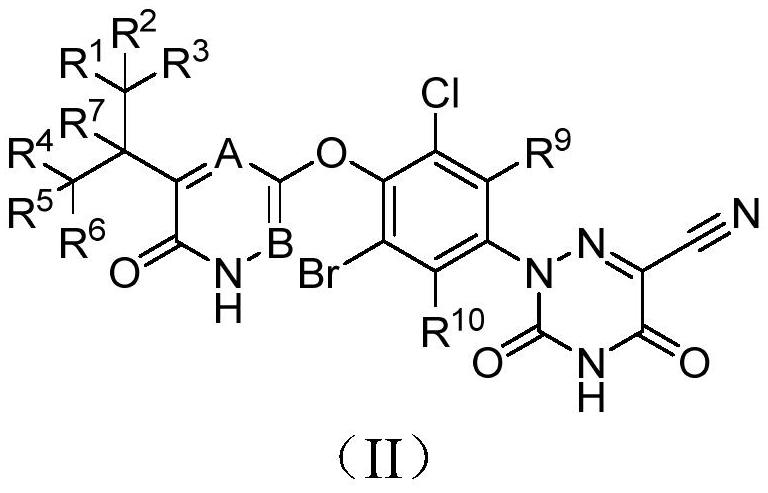
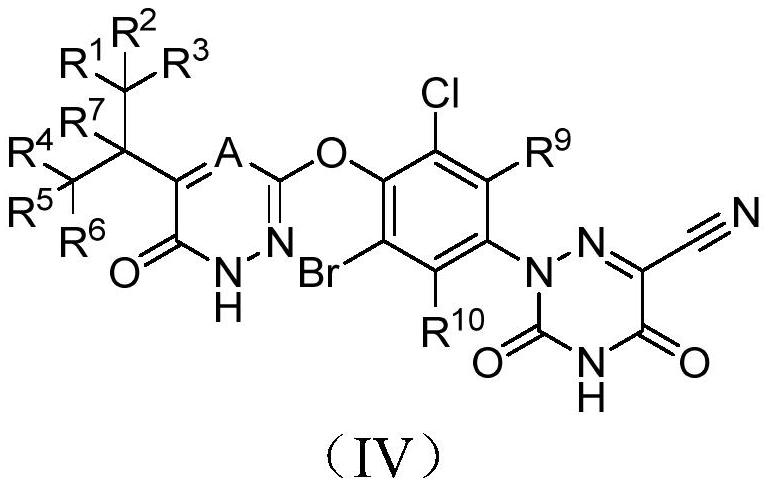
Halogen-substituted phenyl ether compound and application thereof
A technology for compounds and uses, applied in the field of drug preparation, can solve the problems of deteriorating pharmacokinetic properties, inability to prolong half-life, unpredictable sites, etc., and achieve improved pharmacokinetic properties, agonistic activity and agonistic selectivity, Good agonistic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] The synthesis of embodiment 1, compound 2
[0047]
[0048](1) Synthesis of compound 3-bromo-5-chloro-4-((6-chloro-5-bis(trideuteromethyl)methylpyridazin-3-yl)oxy)aniline (compound 2-1)
[0049]
[0050] Synthesis of 3,6-dichloro-4-(1,1,1,3,3,3-hexadeuteriopropan-2-yl)pyridazine (Compound A):
[0051]
[0052] 2-trideuteromethyl-3,3,3-trideuteropropanoic acid A-4 was prepared by literature method (Canadian Journal of Chemistry, 2014, 92, 305). Weigh 2-trideuteromethyl-3,3,3-trideuteropropionic acid (1.4g, 15mmol) into a 100mL three-neck round bottom flask, add 20mL water to it, stir at room temperature to dissolve and clarify. Then 3,6-dichloropyridazine (2.2 g, 15 mmol) was added to the system and stirred at room temperature. Then silver nitrate (2.5 g, 15 mmol) was added to the system, and the system was transferred to an oil bath to raise the temperature, heat and stir the reaction. When the temperature in the system rose to 50°C, concentrated sulfuric ac...
Embodiment 2
[0063] Example 2, 2-(3-bromo-5-chloro-4-((4-deuterium-5-(1,1,1,3,3,3-hexadeuteriopropan-2-yl)-6- Oxy-1,6-dihydropyrazin-3-yl)oxy)phenyl)-3,5-dioxy-2,3,4,5-tetrahydro-1,2,4-triazine-6 - Synthesis of Cyanide (Compound 3)
[0064]
[0065] (1) Compound 3-bromo-5-chloro-4-((6-chloro-5-(prop-2-yl-1,1,1,3,3,3-hexadeuterio)pyrazin-3-yl Synthesis of -4-deuterium)oxy)aniline
[0066]
[0067] Synthesis of 3,6-dichloro-4-deuterium-5-(1,1,1,3,3,3-hexadeuteriopropan-2-yl)pyridazine (compound B):
[0068]
[0069]Weigh the compound 2,3-dichloromaleic anhydride B-1 (8.35g, 50mmol), add it to a 100ml round bottom flask, add 40ml water, add hydrazine hydrate (2.5g, 50mmol), heat to reflux, and keep it warm for 4h , cooled to room temperature, ice-water bath for 30min, filtered, the filter cake was rinsed with 100ml of water, and dried to obtain 5.0g of 4,5-dichloromaleic hydrazide (B-2), yield: 55.3%, MS (ESI )m / e 181.0(M+H) + .
[0070] Synthesis of 4,5-dideuterium-maleic hydr...
Embodiment 3
[0084] Example 3, 2-(3,5-dibromo-4-((6-oxo-5-(prop-2-yl-1,1,1,3,3,3-hexadeuterio)-1,6 -dihydropyrazin-3-yl)oxy)phenyl)-3,5-dioxo-2,3,4,5-tetrahydro-1,2,4-triazine-6-cyano (compound 14 )Synthesis
[0085]
[0086] (1) 3,5-dibromo-4-((6-chloro-5-(prop-2-yl-1,1,1,3,3,3-hexadeuterio)pyridazin-3-yl)oxy ) Synthesis of aniline:
[0087]
[0088] Compound 3,6-dichloro-4-(propan-2 base-1,1,1,3,3,3-hexadeuterio)pyridazine A (330mg, 1.67mmol) was dissolved in 5ml DMSO, and compound 4- Amino-2,6-dibromophenol (559mg, 2.09mmol), K2CO3 (923mg, 6.68mmol) was added under stirring, Ar was replaced 3 times, heated to 90°C, reacted for 3h, cooled, added water, extracted with ethyl acetate, The organic layer was washed with water and saturated brine respectively, dried over anhydrous sodium sulfate, concentrated to dryness under reduced pressure, separated and purified by column chromatography (petroleum ether / ethyl acetate=5:1) to obtain compound 3,5-dibromo-4- ((6-Chloro-5-(propan-2-yl-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com