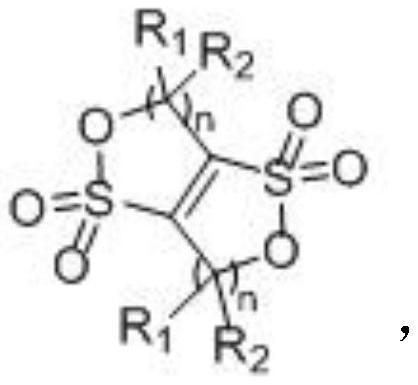
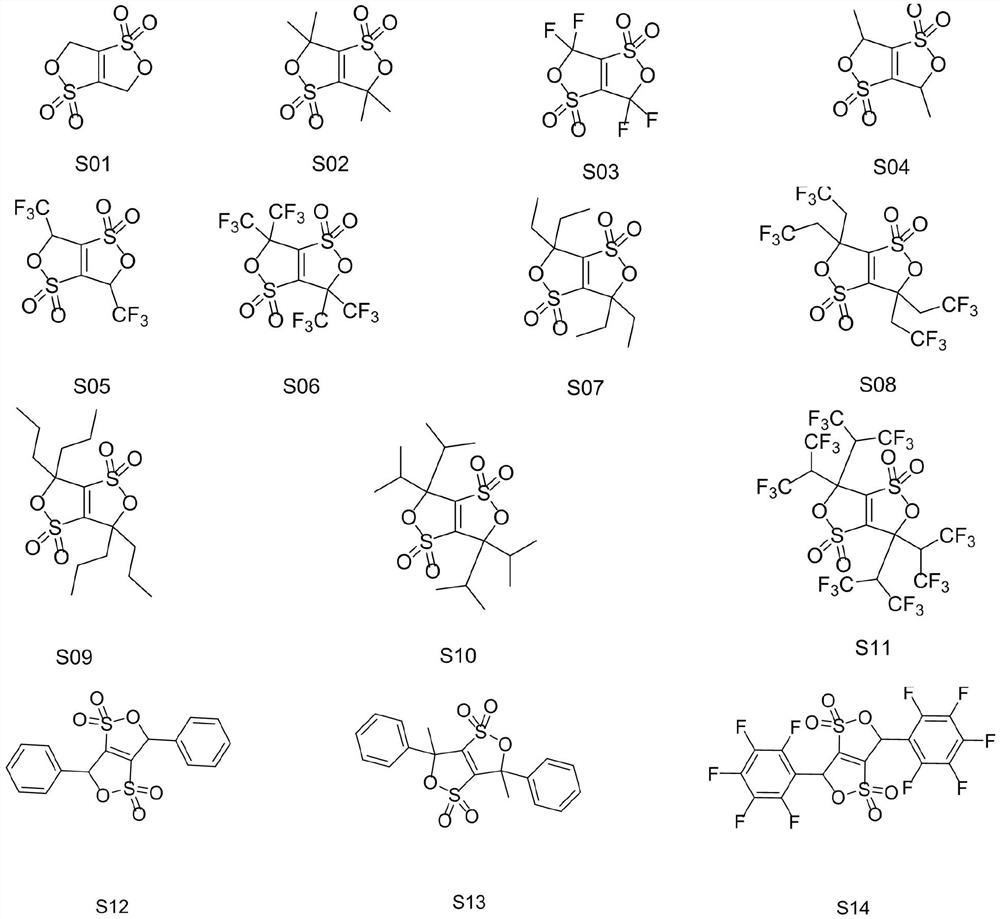
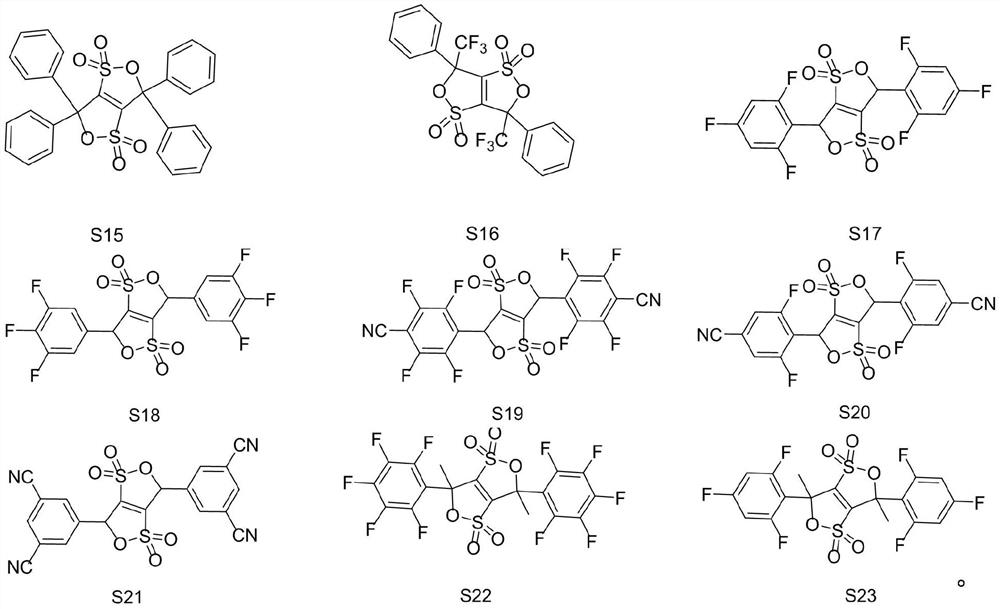
A kind of cyclic sulfonate lithium ion battery electrolyte additive, its preparation method and application
An electrolyte additive, lithium-ion battery technology, applied in the manufacture of electrolyte batteries, secondary batteries, non-aqueous electrolyte batteries, etc., can solve the problems of reduced battery performance, increased micronization of negative electrode materials, and hindered battery electrochemical reactions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] (1) Preparation of Intermediate 1
[0026]
[0027] Raw material 1 can be purchased commercially or prepared by the preparation method of patent CN102249861;
[0028] Add 123g of raw material 1, 50.4g of sodium sulfite and 140.05g of sodium bisulfite into a 2L three-necked flask, add 550g of deionized water and 150g of ethanol, stir vigorously, raise the temperature to reflux of the system, reaction time is 18h, desolventize under reduced pressure until there is no fraction , the black residue was acidified with ethanol solvent, passed through hydrogen chloride gas, and a large amount of NaCl solid was precipitated, and the insoluble matter was filtered off, and the obtained filtrate was further desolventized until there was no fraction, and 153g of intermediate 1 was obtained, containing part of the solvent, for subsequent use;
[0029] (2) Preparation of S01
[0030]
[0031] Add chlorobenzene to the intermediate 1 obtained above, heat up to 132°C to reflux, an...
Embodiment 1-12 and comparative example 1-6
[0054] The preparation method of the lithium battery of embodiment 1-12 and comparative example 1-6:
[0055] (1) Preparation of positive electrode sheet
[0056] LiCoO 2 Cathode material as an example: the positive electrode LiCoO 2 Powder, carbon black (particle size 1000nm), polyvinylidene fluoride (PVDF) and N,N-dimethylpyrrolidone (NMP) were mixed to make a uniform slurry, and the slurry was uniformly coated on an aluminum foil (15 μm) set Fluid, dried, rolled to obtain LiCoO 2 Positive electrode material; baked at 120°C for 12 hours, in the dried electrode sheet, LiCoO 2 Account for 94% of the total coating, binder accounts for 4%, and carbon black accounts for 2%. Gained pole pieces are cut into diameters of 8mm discs as positive electrodes, and other positive electrode materials are prepared in the same way;
[0057] (2) Negative sheet preparation
[0058] Take artificial graphite anode material as an example: artificial graphite, polyvinylidene fluoride (PVDF) an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com