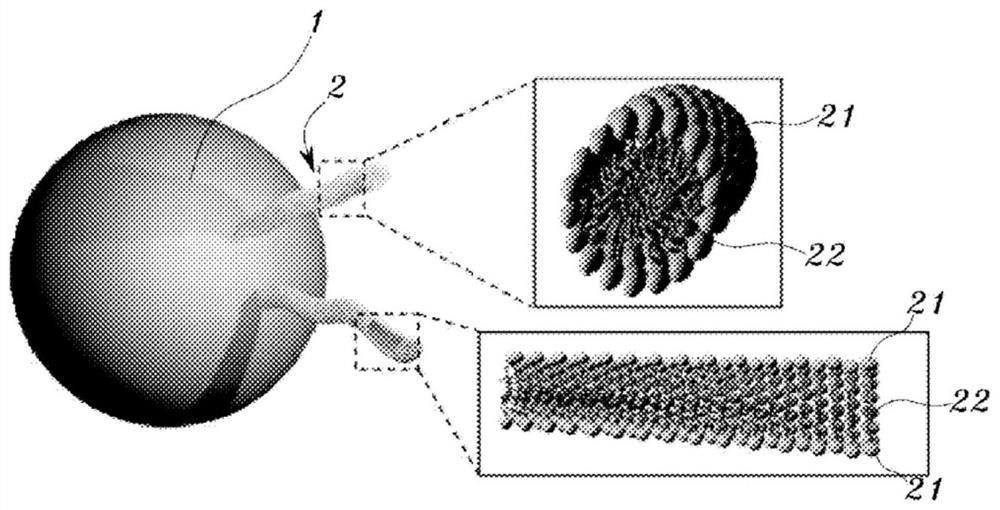
Nanoparticle composite showing improved endocytosis efficiency through surface modification using lipid and manufacturing method therefor
A nanoparticle and manufacturing method technology, applied in the direction of liposome delivery, other methods of inserting foreign genetic materials, medical preparations of non-active ingredients, etc., can solve the problems of decreased stability and insufficient achievement, and achieve large-scale The effect of mass production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Production of Nanoparticle Composite
[0057] 1. Formation of Nanoparticles (NPs)
[0058] After dissolving albumin (Human serum albumin) in distilled water to a concentration of 20mg / mL, use 0.2M NaOH to adjust the pH to 8 to prepare an albumin solution, and then add 100% ethanol to the above albumin solution at a rate of 1mL / min Titrate. Next, 10 μL of 4% glutaraldehyde (glutaraldehyde) was added and ethanol was evaporated overnight under light-shielding conditions, and after centrifugation was performed at 13200 rpm for 10 minutes, unparticled albumin was removed with a pipette, Next, use a pipette to obtain the supernatant (nanoparticles (NPs)) except micropellets (micropellets) after redispersing with phosphate buffered saline (PBS) and performing centrifugation under the conditions of 3000rpm and 5min (in addition, When performing the fluorescence test, react the fluorescent dye (dye) and nanoparticles that meet the requirements overnight at room temperature, t...
Embodiment 2
[0066] Characteristic Confirmation of Nanoparticle Complex
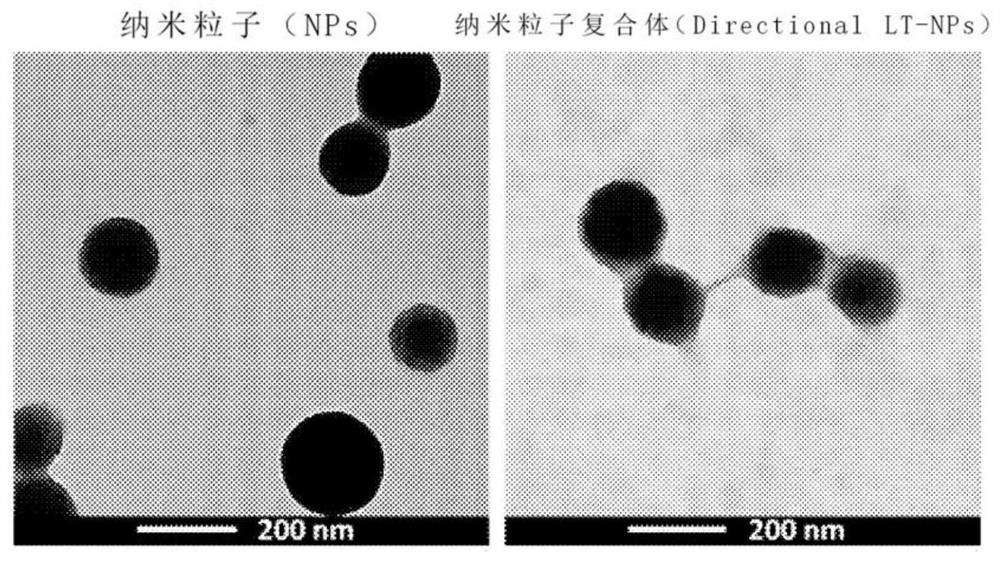
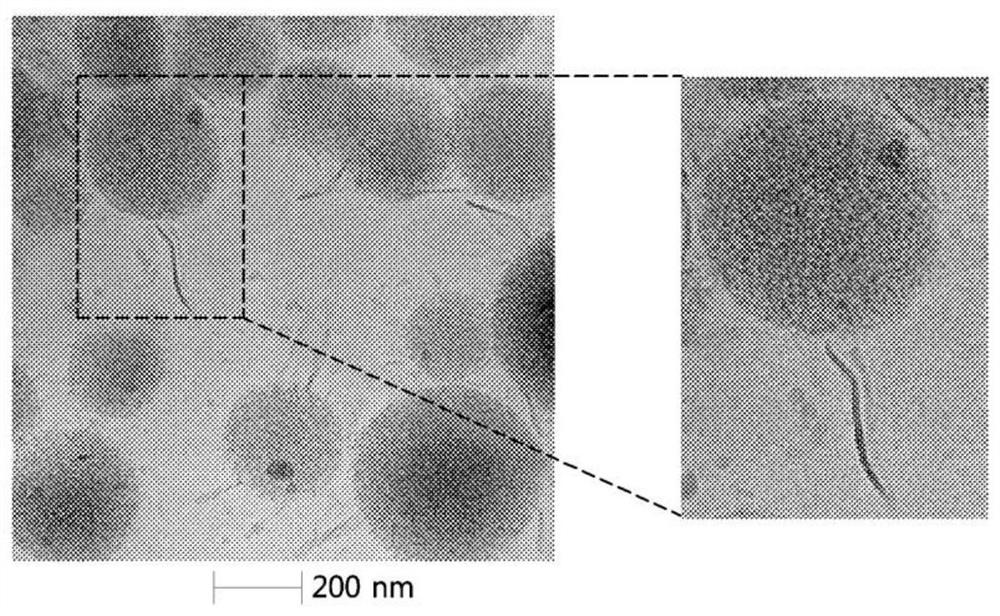
[0067] 1. Utilize transmission electron microscope (TEM) to measure the nanoparticles (NPs) formed in 1 of embodiment 1 and the nanoparticle complex (Directional LT-NPs) formed in (2) in 4 of embodiment 1 , the result is as figure 2 Shown, and utilize frozen transmission electron microscope (Cyro-TEM) to measure above-mentioned nanoparticle complex and lipid structure body, its result is as follows respectively image 3 as well as Figure 4 shown.
[0068] 2. pass figure 2 It was confirmed that the surface of the nanoparticle was smooth, but the nanoparticle complex showed an uneven surface due to the lipid structure attached to the surface of the nanoparticle. Additionally, by image 3 It can be confirmed that a tubular lipid structure is attached to the surface of the nanoparticle, by Figure 4 It was clearly confirmed that the above-mentioned lipid structure had a tubular form. Additionally, by image 3...
Embodiment 3
[0069] Evaluation of Cellular Uptake Efficiency of Nanoparticle Complex
[0070] 1. In order to evaluate the cellular uptake efficiency of the nanoparticle (NPs) formed in 1 of Example 1 and the nanoparticle complex (Directional LT-NPs) formed in (2) of Example 1 Flow cytometry (Flowcytometry) for analysis, the results are as follows Figure 5 shown, and imaged using a confocal microscope, the results are shown in Image 6 shown. The analysis by flow cytometry is performed by using Alexa 488 fluorescent dye (dye) labeled nanoparticles and nanoparticle complexes on A549 cells (1 × 10 4 ), while the analysis using confocal microscopy was performed by using Cy5.5 fluorescent dye (dye)-labeled nanoparticles and nanoparticle complexes to stain nuclei with DAPI and use phalloidin (phalloidin) A549 cells stained for cytoskeleton (1×10 5 ) is executed in the manner of processing.
[0071] 2. In addition, in order to compound the nanoparticles (NPs) formed in 1 of Example 1 and t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com