Glutathione response type double-loading medicine polymer micelle and preparation method and application thereof
A technology of glutathione and polymers, applied in the field of biomedical engineering materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Example 1: Synthesis of dihydroxypolycaprolactone (PCL) with disulfide bonds
[0089] Dissolve cystamine and ε-caprolactone with a disulfide bond in dry toluene, slowly add the catalyst stannous isooctanoate dropwise for 20 minutes under anhydrous and anaerobic conditions, and after the dropwise addition, place at 110°C in a nitrogen atmosphere Condensation and reflux reaction for 18 hours, magnetic stirring during the reaction, the rotation speed was 300rpm; after the reaction was completed, the toluene solvent was removed by rotary evaporation at 40°C, and then precipitated with cold ether, and then vacuum-dried at 40°C for 12h to obtain dihydroxypolycaprolactone with disulfide bonds Esters (PCL).
[0090] The mol ratio of described cystamine, epsilon-caprolactone, stannous isooctanoate is 1:132:0.05; The quality of described cystamine is 200mg; The total volume of described dried toluene is 10mL; Used cold The volume ratio of diethyl ether and reaction system soluti...
Embodiment 2
[0091] Example 2: Synthesis of dihydroxypolycaprolactone (PCL) with disulfide bonds
[0092] Dissolve cystamine and ε-caprolactone with a disulfide bond in dry toluene, slowly add the catalyst stannous isooctanoate dropwise for 50 minutes under anhydrous and anaerobic conditions, and after the dropwise addition, place at 110°C in a nitrogen atmosphere Condensation and reflux reaction for 24 hours, magnetic stirring during the reaction, the rotation speed was 600rpm; after the reaction was completed, the toluene solvent was removed by rotary evaporation at 60°C, and then precipitated with cold ether, and then vacuum-dried at 40°C for 24h to obtain dihydroxypolycaprolactone with disulfide bonds Esters (PCL).
[0093] The mol ratio of described cystamine, epsilon-caprolactone, stannous isooctanoate is 1:132:0.1; The quality of described cystamine is 100mg; The total volume of described dried toluene is 10mL; Used cold The volume ratio of diethyl ether to the reaction system solu...
Embodiment 3
[0094] Embodiment 3: NMR characterization of dihydroxy polycaprolactone (PCL) with disulfide bonds
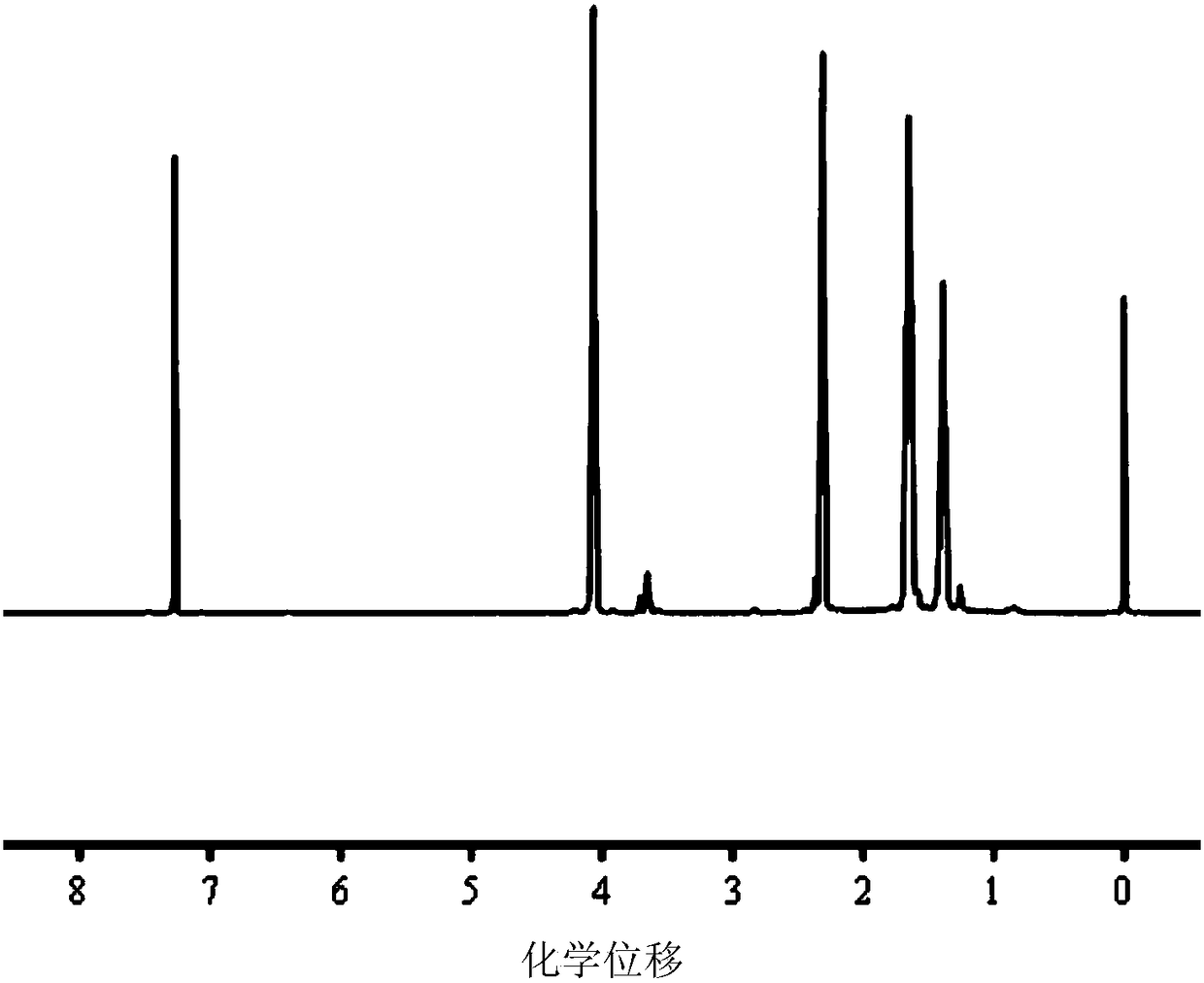
[0095] The dihydroxy polycaprolactone (PCL) having a disulfide bond obtained in Example 1 was dissolved in deuterated chloroform for H NMR characterization. Such as figure 1 As shown, the peaks with chemical shifts at 4.08, 2.33, 1.40, and 1.67ppm are the characteristic peaks of the five methylene hydrogens after caprolactone ring opening, and 4.08ppm represents the characteristic peaks of the methylene hydrogens connected with oxygen , 2.33ppm represents the characteristic peak of methylene hydrogen linked to carbonyl; the peak at 3.55ppm is the hydrogen peak of methylene linked to cystamine. figure 1 The results proved that the step reaction successfully synthesized dihydroxypolycaprolactone (PCL) with disulfide bonds.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com