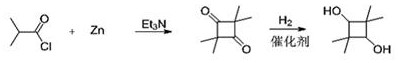
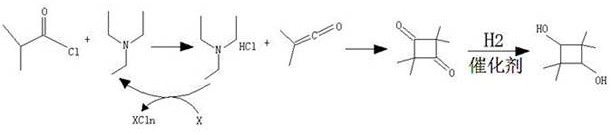
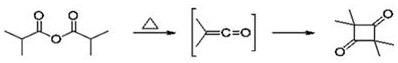
A kind of preparation method of 2,2,4,4-tetramethyl-1,3-cyclobutanediol
A technology of cyclobutanediol and tetramethyl, which is applied in the field of preparation of 2,2,4,4-tetramethyl-1,3-cyclobutanediol, can solve the problems of low conversion rate of cracking reaction and triethylamine Hydrochloride treatment and recovery are difficult, and it is difficult to further improve problems such as
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Add 210 g of isobutyryl chloride (1.97 mol) and 1.2 L of ethyl acetate into a dry 2 L dry three-neck flask, stir mechanically, add 2 g of triethylamine (0.019 mol) and 65 g of zinc powder (0.99 mol) Control the temperature of the reaction solution below 40°C, stir for 30 min, then raise the temperature to reflux at 68°C, the reaction time is 20 h, and the reaction is over.
[0044] Cool the reaction solution below 30°C, remove inorganic salts by filtration, add 200ml of 0.1M dilute hydrochloric acid to the mother liquor to wash twice, wash once with 200ml of saturated sodium chloride, dry with 20g of anhydrous sodium sulfate, filter, and distill the mother liquor under reduced pressure Remove the solvent, control the temperature of the water bath at about 30°C~40°C, a large amount of light yellow solids will precipitate until no ethyl acetate flows out after distillation, add 300 ml of petroleum ether to the residue, stir and cool to 0°C, filter, collect the solids, and ...
Embodiment 2
[0047] In a dry 2 L dry three-necked flask, add 210 g of isobutyryl chloride (1.97 mol) and 1.2 L of ethyl acetate, stir mechanically, add 2 g of triethylamine (0.019 mol) and 63 g of zinc powder (0.81 mol) to control The temperature of the reaction solution was below 40°C. After stirring for 30 minutes, the temperature was raised to 68°C and refluxed. The reaction time was 20 hours, and the reaction was completed.
[0048]Cool the reaction solution below 30°C, remove inorganic salts by filtration, add 200ml of 0.1M dilute hydrochloric acid to the mother liquor to wash twice, wash once with 200ml of saturated sodium chloride, dry with 20g of anhydrous sodium sulfate, filter, and distill the mother liquor under reduced pressure Remove the solvent, control the temperature of the water bath at about 30°C~40°C, a large amount of light yellow solids will precipitate until no ethyl acetate flows out after distillation, add 300 ml of petroleum ether to the residue, stir and cool to 0°...
Embodiment 3
[0051] In a dry 2 L dry three-neck flask, add 210 g isobutyryl chloride (1.97 mol) and 1.2 L ethyl acetate, stir mechanically, add 2 g triethylamine (0.019 mol) and 78.5 g zinc powder (1.2 mol) Control the temperature of the reaction solution below 40°C, stir for 30 min, then raise the temperature to reflux at 68°C, the reaction time is 20 h, and the reaction is over.
[0052] Cool the reaction solution below 30°C, remove inorganic salts by filtration, add 200ml of 0.1M dilute hydrochloric acid to the mother liquor to wash twice, wash once with 200ml of saturated sodium chloride, dry with 20g of anhydrous sodium sulfate, filter, and distill the mother liquor under reduced pressure Remove the solvent, control the temperature of the water bath at about 30°C~40°C, a large amount of light yellow solids will precipitate until no ethyl acetate flows out after distillation, add 300 ml of petroleum ether to the residue, stir and cool to 0°C, filter, collect the solids, and get TMCB ti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com