Enzymatic synthesis method of bimatoprost intermediate
A technology of bimatoprost and synthetic method, which is applied in the field of biopharmaceuticals, can solve the problems of unfriendly environment and low chiral purity, and achieve the effects of simple and convenient operation, high optical purity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
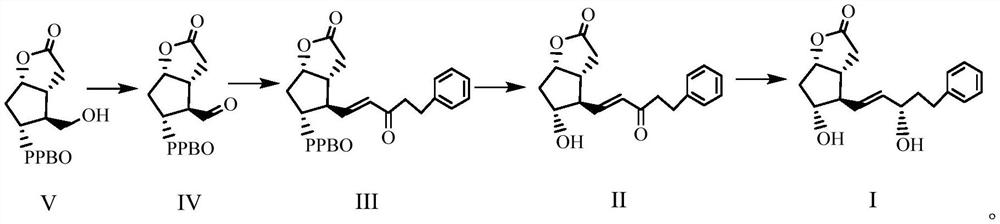
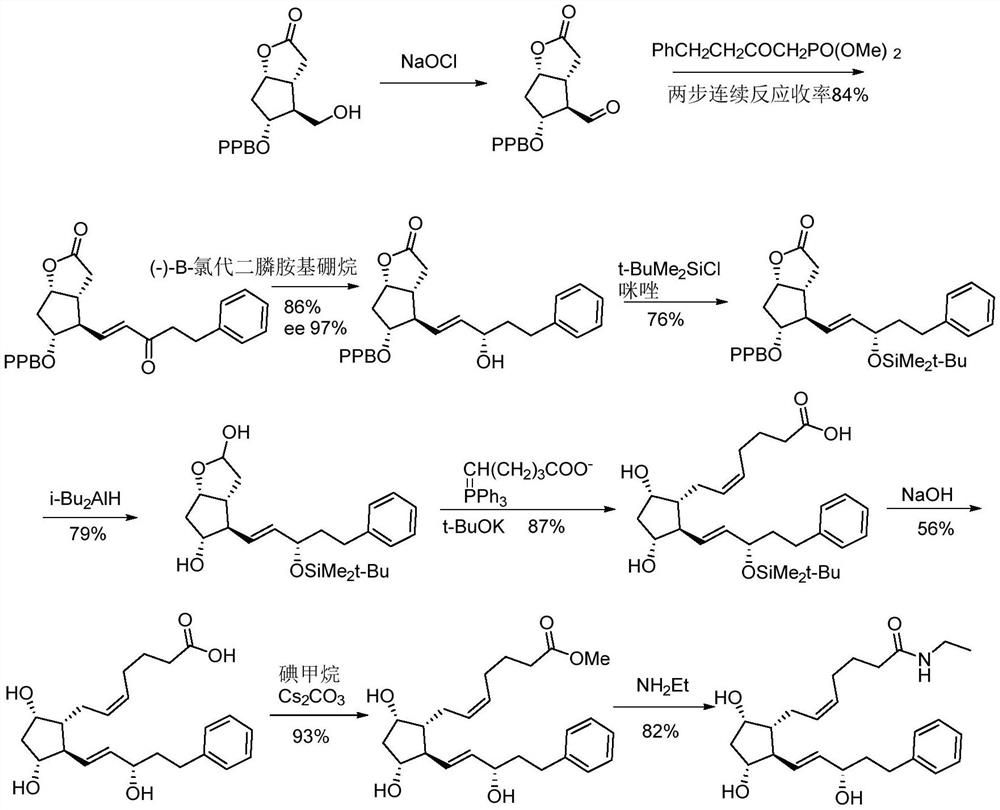
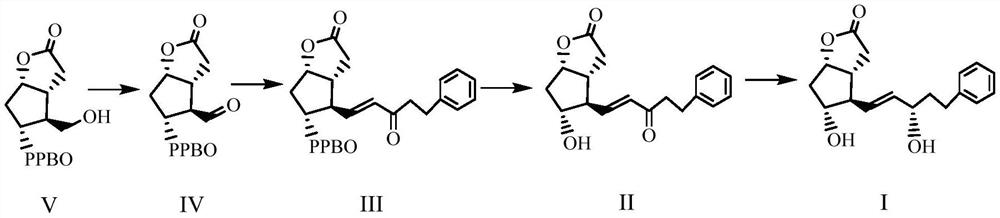
[0035] In the reaction flask, add oxalyl chloride (8.0ml, 0.08mol) and dichloromethane (160ml) sequentially under nitrogen protection, cool to -75~-65°C, add DMSO (16.0ml, 0.22mol) dropwise, and keep the temperature at -75 After stirring for 1 h at ~-65°C, slowly add compound V (16.0 g, 58 mmol) in dichloromethane solution (140 ml) dropwise. After continuing to stir for 1 h, slowly add triethylamine (40 ml) dropwise, and heat up to -50 React at ℃ for 2 hours, monitor until the reaction of the raw materials is complete, quench the reaction with 10% sodium dihydrogen phosphate aqueous solution (200ml), separate the liquids after stirring, wash the organic phase with saturated sodium chloride solution (100ml×3), and wash with anhydrous sulfuric acid After sodium drying and filtration, the dichloromethane solution of compound VI was obtained for future use (among them, the purity of compound VI was 98.5%, the yield was 92.7%, and it is used now).
Embodiment 2
[0037] In the reaction flask, lithium chloride (8.6g, 0.25mol), (2-oxo-4-phenylbutyl) dimethyl phosphate (24.0g, 0.094mol) and acetonitrile (170ml) were added successively under nitrogen protection. , cooled to -10~0°C, DIPEA (25ml, 0.14mol) was added dropwise, and after stirring evenly, the dichloromethane solution (500ml, 0.09 mol), add in about 0.5h, react for about 2-3h, monitor until the reaction is complete, wash with saturated sodium chloride solution (160ml×3), filter after drying with anhydrous sodium sulfate, spin the filtrate to get a light yellow solid , adding petroleum ether (30ml), beating and filtering to obtain white powder compound III (23.5g, purity 94.8%, yield 95.2%).
Embodiment 3
[0039]Add compound III (20.8g, 51.2mmol) and methanol (200ml) in turn to the reaction flask. After stirring and dissolving, add potassium carbonate powder (6.4g, 44.8mmol), control the temperature at 20-25°C, and react for about 3.5h , monitor until the reaction is complete. Add 1mol / L hydrochloric acid (about 75ml) to the obtained reaction solution to adjust the pH of the mixed solution to 6, then add water (120ml), extract with methyl tert-butyl ether (320ml×4), combine the organic phases, add methanesulfonate Acid (960l) was stirred for 45min, washed successively with saturated sodium bicarbonate solution (320ml×2) and saturated sodium chloride solution (320ml), dried over anhydrous sodium sulfate and filtered, and the filtrate was evaporated to dryness under reduced pressure to obtain a colorless oil compound II (16.5 g, 98.5% purity, 96.4% yield).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com