Degradable metal uterine cavity stent, release system and use method
A release system, uterine cavity technology, applied in stents, medical science, prostheses, etc., can solve the problems of re-adhesion technology, monopoly, poor medical device effect, etc., to reduce the burden of medical treatment, prevent adhesion, and stabilize mechanical support. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: Structural design of a novel degradable metal intrauterine stent and its release system.
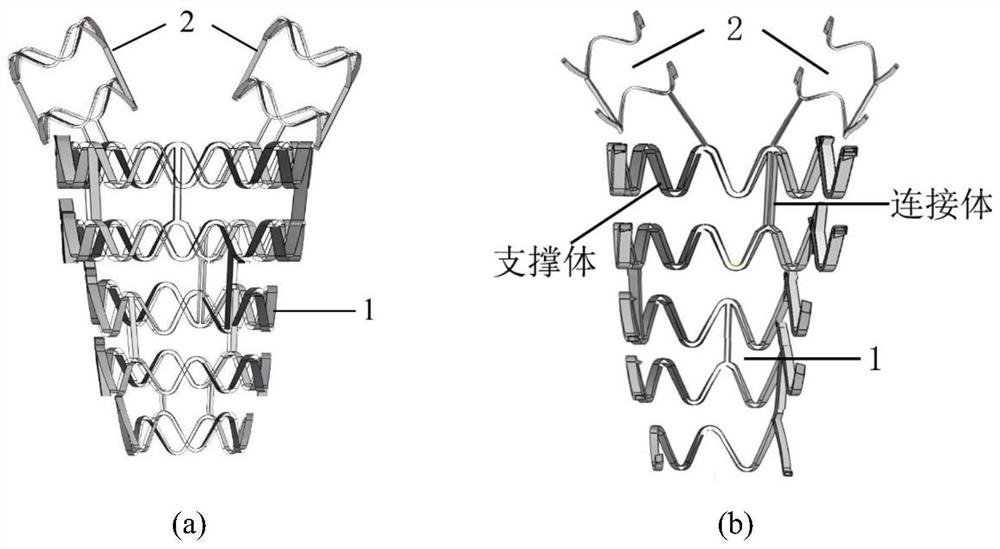
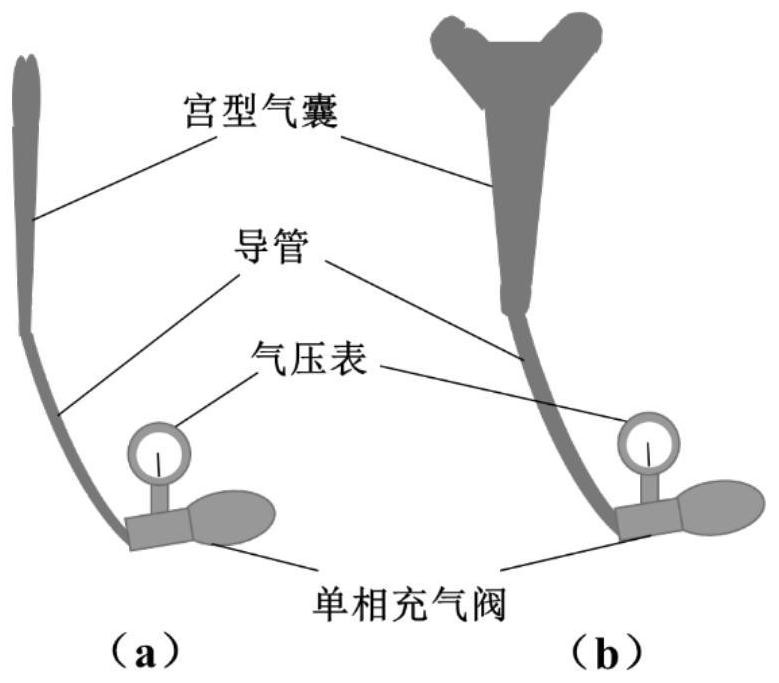
[0027] like figure 1 As shown, the device structure of the present invention includes an intrauterine stent and a release system for the treatment of uterine adhesions.
[0028] The structure of the new degradable metal uterine stent and release system is as follows: the thickness of the uterine stent is 50-100 μm. ( figure 1 ) According to the size of the human uterus, the post-expanded uterine cavity stent is 3-8 cm high, 2-5 cm wide, and 0.2-1 cm thick, and its shape conforms to the uterine cavity. The intrauterine stent consists of 1 and 2 parts ( figure 1 a), acting on the uterine cavity and the uterine horn, respectively, part 2 is connected to part 1 by welding. It can be seen from the cross-sectional view of the uterine stent ( figure 1 b), the part of the stent away from the cervix has a thicker thickness and a higher density of the support body, which can...
Embodiment 2
[0032] Example 2: Preparation of Novel Degradable Metal Uterine Stent
[0033] The preparation steps of the new degradable metal uterine branch are: selection of medical degradable metal uterine stent material→laser cutting→grinding→welding→electropolishing.
[0034] In the first step, Mg-3wt% Zn, Zn-0.8wt% Li and pure Fe tubes are selected as medical degradable metal intrauterine stent materials, the diameter of the tube is 2-5cm, and the tube thickness is 50-150μm; the yield strength of the above material is 220-400MPa, tensile strength is 350-600MPa, elongation is 20-90%.
[0035] In the second step, the selected tube is laser-cut into an intrauterine stent. After grinding, the different parts are assembled by welding. Finally, the uterine stent obtained by electropolishing is 3-8 cm high, 2-5 cm wide, and 50-150 μm thick. The radial support force of the above-mentioned degradable metal uterine stent was measured to be 15-26 kPa, which can play an effective mechanical supp...
Embodiment 3
[0036] Example 3: In vivo implantation experiment of degradable intrauterine stents in animals
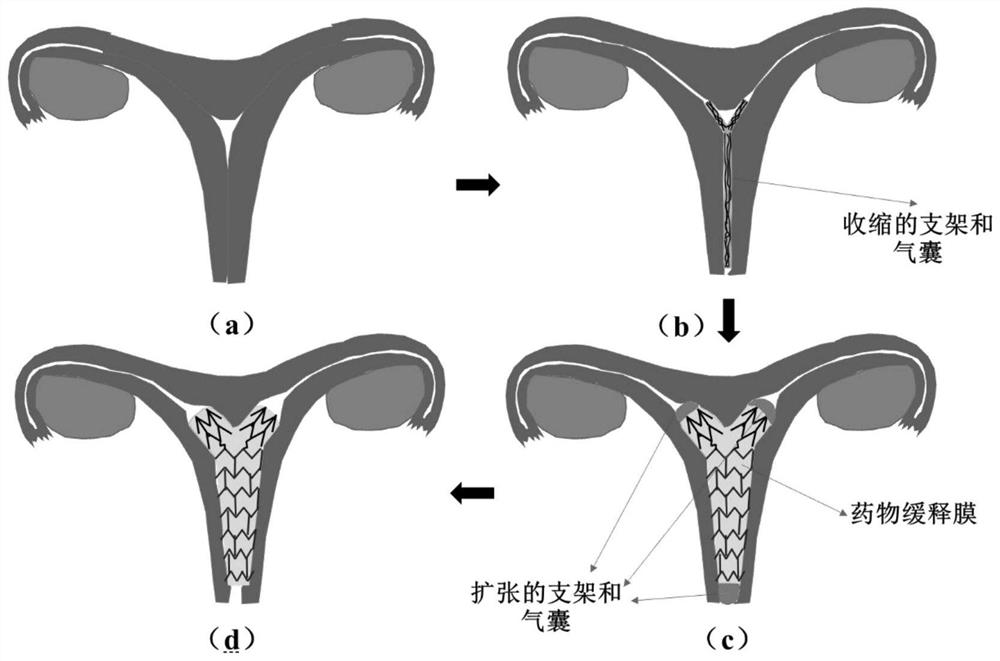
[0037] Adult sexually mature female Shanghai White pigs with uterine adhesions were selected as degradable uterine stent implantation objects. According to the size of the uterus of adult female Shanghai White pigs (length 1-1.5 cm, width 1.5-3 cm), a suitable size of degradable stent was designed and prepared. Intrauterine stent and release system, the degradable stent is made of Zn-0.8Li alloy. The intrauterine stent and release system can be placed into the uterine cavity through toothless forceps in a contracted state, and then expanded into a uterine-like shape through the release system, and then removed for observation 1, 2, 3, and 6 months after implantation.
[0038] Postoperative hysteroscopic observation showed that the shape of the degradable uterine stent conformed to the uterine cavity after expansion, and formed an effective support and isolation for the uterine angl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Yield strength | aaaaa | aaaaa |
| Tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com