Olaparib intermediate and preparation method of olaparib
An intermediate and solvent technology, applied in the field of intermediates and preparation, can solve the problems of unfavorable industrial production, expensive raw materials, difficult post-processing, etc., and achieve the effects of less three wastes, simple post-processing, and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
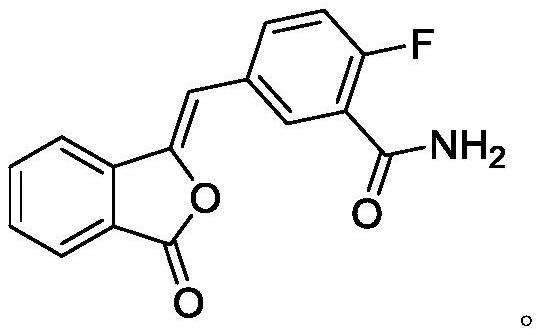
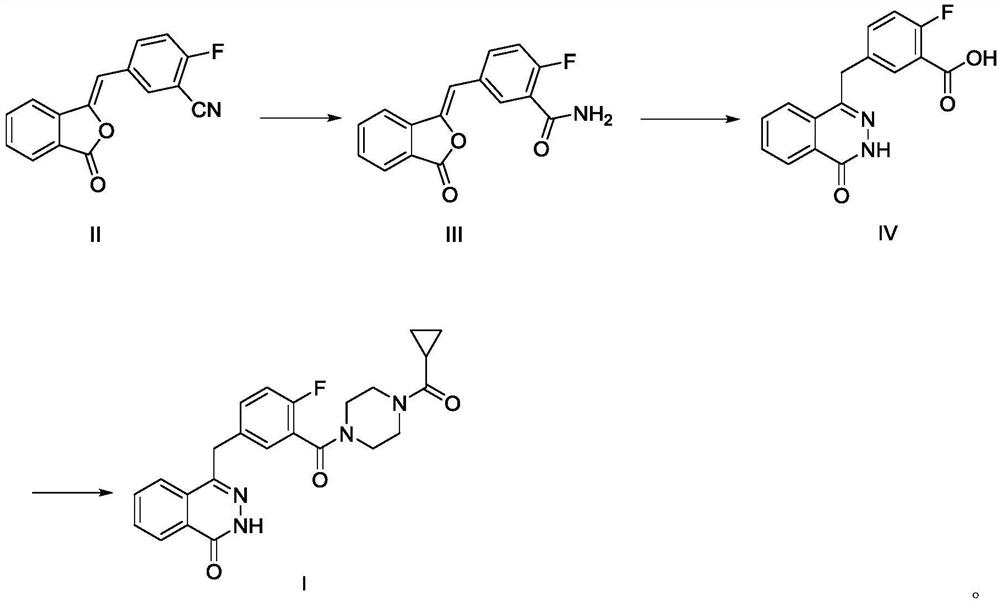
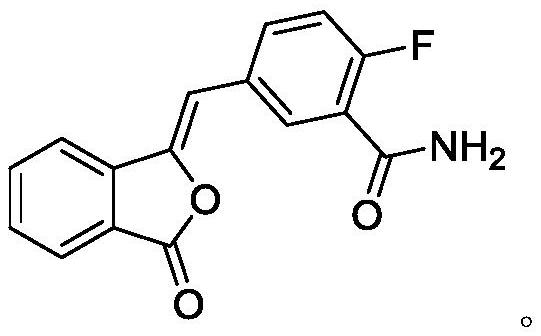
[0029] (1) Preparation of compound III
[0030] Add 50g of compound II and 500g of water into a 1L four-neck flask, stir, heat up to 25-30°C, quickly add aqueous sodium hydroxide solution (9g sodium hydroxide + 250g water), keep warm for 15min, the system gradually dissolves, TLC monitors the compound I reacted completely. Adjust the pH to 1-2 with 6M hydrochloric acid, let stand to separate the liquids; wash the organic phase twice with 1L saturated brine, and dry with anhydrous sodium sulfate; concentrate under reduced pressure until solvent-free. Add 45g of tetrahydrofuran and 250g of water, at this time the system precipitates an oily substance; cool down to 20-25°C, the oily substance becomes viscous, add a mixed solvent of ethyl acetate (50mL) and petroleum ether (100mL) dropwise; after stirring for 1h A large amount of solid was precipitated; filtered with suction, the filter cake was rinsed with water, and the filter cake was dried under reduced pressure at 50-60°C un...
Embodiment 2
[0036] (1) Preparation of compound III
[0037] Add 50g of compound II and 500g of DMF into a 1L four-neck flask, stir, raise the temperature to 20-25°C, quickly add 9g of potassium hydroxide, keep it warm for 15min, the system gradually dissolves, and TLC monitors that the reaction of compound I is complete. The pH was adjusted to 1-2 with 6M hydrochloric acid, and the liquids were separated; the organic phase was washed twice with 1 L saturated brine, and dried with anhydrous sodium sulfate; concentrated under reduced pressure until solvent-free. Add 45g of tetrahydrofuran and 250g of water, and the system precipitates an oily substance at this time; continue to add a mixed solvent of ethyl acetate (50mL) and petroleum ether (100mL); after stirring for 1h, a large amount of solids are precipitated; Dry the material under reduced pressure at -60°C to constant weight to obtain 48.5 g of solid with a purity of 98.6%.
[0038] (2) Preparation of Compound IV
[0039] Add 20g of...
Embodiment 3
[0043] (1) Preparation of compound III
[0044] Add 50g of compound II and 500g of acetonitrile into a 1L four-neck flask, stir, raise the temperature to 25-30°C, add 18g of pyridine, and keep it warm for 15 minutes. The system gradually dissolves, and the reaction of compound I is monitored by TLC. The pH was adjusted to 1-2 with 6M hydrochloric acid, and the liquids were separated; the organic phase was washed twice with 1 L saturated brine, and dried with anhydrous sodium sulfate; concentrated under reduced pressure until solvent-free. Add 45g of acetonitrile and 250g of water, at this time the system precipitates an oily substance; cool down to 20-25°C, the oily substance becomes viscous, add dropwise a mixed solvent of ethyl acetate (50mL) and petroleum ether (100mL); after stirring for 1h A large amount of solid was precipitated; filtered with suction, the filter cake was rinsed with water, and the filter cake was dried under reduced pressure to constant weight to obtain...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com