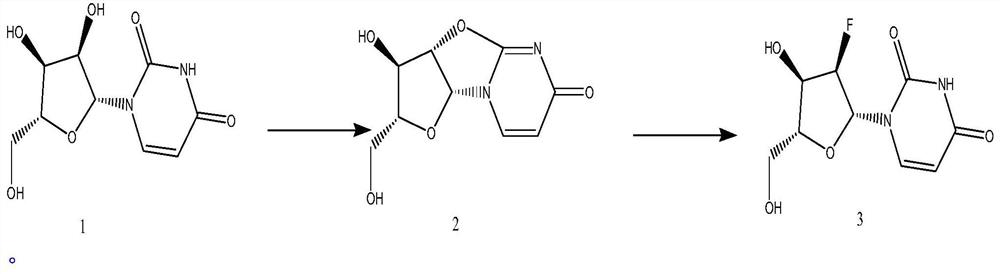
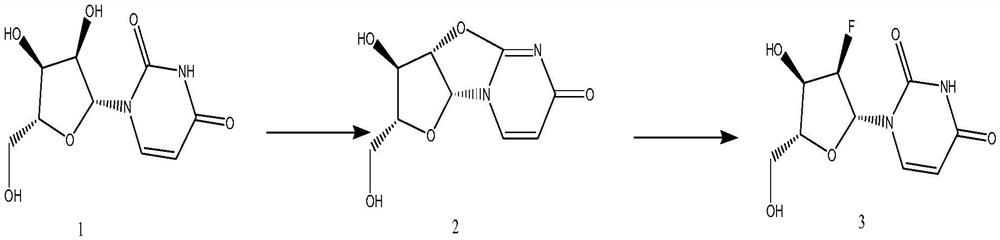
Synthetic method of 2'-Deoxy-2'-fluorouridine
A synthesis method and technology of deoxyuridine, applied in chemical instruments and methods, sugar derivatives, organic chemistry and other directions, can solve the problems of high equipment requirements, high degree of risk, unsuitable for scale-up production, etc., and achieve high reaction conversion rate, good yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] The synthesis of embodiment 1 compound 2
[0021] Add 69.0g uridine to the reaction flask, add 69.0g (1.1eq) diphenyl carbonate, add 163.0g DMF, start stirring, and gradually raise the temperature to 58-62°C. During the heating process, the solid dissolves continuously. At about 60°C, Solution clarification After the solution is clarified, control the temperature at 58-62°C, then add 1.7g of sodium bicarbonate and gradually raise the temperature to 78-82°C. After the reaction is completed, naturally cool down to 20°C-30°C, stir for 1-3 hours, and filter. The filter cake was dried to obtain 57.2g of compound 2, yield: 89.5%, purity: 99.57%.
Embodiment 2
[0022] The synthesis of embodiment 2 compound 3
[0023] Add 20g of compound 2 to the reaction flask, add 12.5g of highly active anhydrous potassium fluoride, 0.2g of boron trifluoride ether, 200ml of DMF, heat up to 120°C for 12 hours, filter after the reaction is completed, concentrate the filtrate, and use tetrahydrofuran and ethyl acetate The ester was recrystallized to obtain 11.6 g of 2'-fluoro-2'-deoxyuridine, with a yield of 53.2% and a purity of 98.2%.
Embodiment 3
[0024] The synthesis of embodiment 3 compound 3
[0025] Add 20g of compound 2 to the reaction flask, add 12.5g of highly active anhydrous potassium fluoride, 0.2g of boron trifluoride ether, and 200ml of chlorobenzene, heat up to 130°C for 12 hours, filter after the reaction is completed, concentrate the filtrate, and use tetrahydrofuran and Recrystallized from ethyl acetate to obtain 10.8 g of 2'-fluoro-2'-deoxyuridine with a yield of 49.8% and a purity of 99.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com