Dialysis method of recombinant human tissue type plasminogen kinase derivative inclusion body enhancement solution, and application
A technology of fibrinolytic enzyme and inclusion body, which is applied in the direction of biochemical equipment and methods, enzymes, peptidases, etc., can solve the problems of less solution treatment, long dialysis time, waste of materials, etc., shorten the dialysis time and improve the dialysis efficiency , Improve the effect of clarity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The preparation of embodiment 1 solution
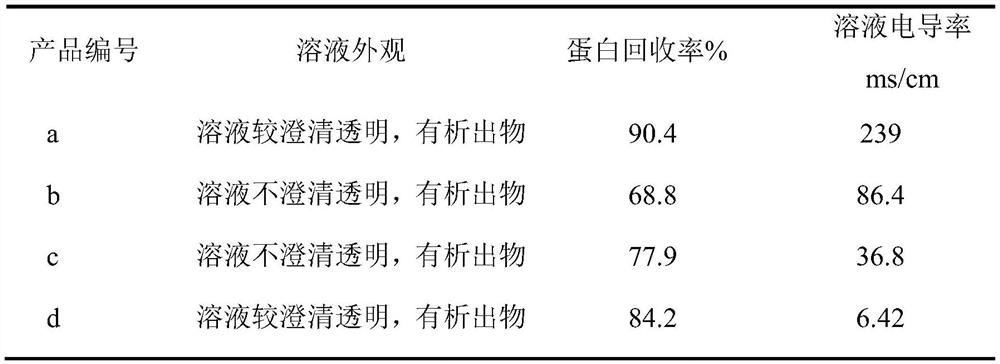
[0029] (1) Preparation of solubilized dialysis inner fluid: prepare solubilizing solution (6mol / L guanidine hydrochloride, 50mmol / L Tris, 1mmol / LEDTA·Na 2 , pH is 8.6); take rPA inclusion body and join in the enhancement solution, inclusion body and enhancement solution are mixed according to the ratio of 1.5kg: 30L, then add dithiothreitol (DTT) to it to concentration to be 40mmol / L, use Finely adjust the pH of the NaOH solution to 8.6, stir for 2 hours, and then adjust the pH to 3.0 with dilute hydrochloric acid to obtain the solubilized dialysis inner solution, which is set aside.
[0030] (2) Preparation of external dialysis fluid:
[0031] Dialysis fluid 1 (4mol / L urea, 1mmol / L EDTA·Na 2 , pH3.0);
[0032] Dialysis fluid 2 (5mol / L urea, 1mmol / L EDTA·Na 2 , pH3.0);
[0033] Dialysis fluid 3 (6mol / L urea, 1mmol / L EDTA·Na 2 , pH3.0);
[0034] Dialysis external fluid 4 (7mol / L urea, 1mmol / L EDTA·Na 2 , pH3.0);
[0035...
Embodiment 2
[0038] Example 2 Using an ultrafilter to dialyze the recombinant human tissue-type plasmin kinase derivative inclusion body enhancement solution
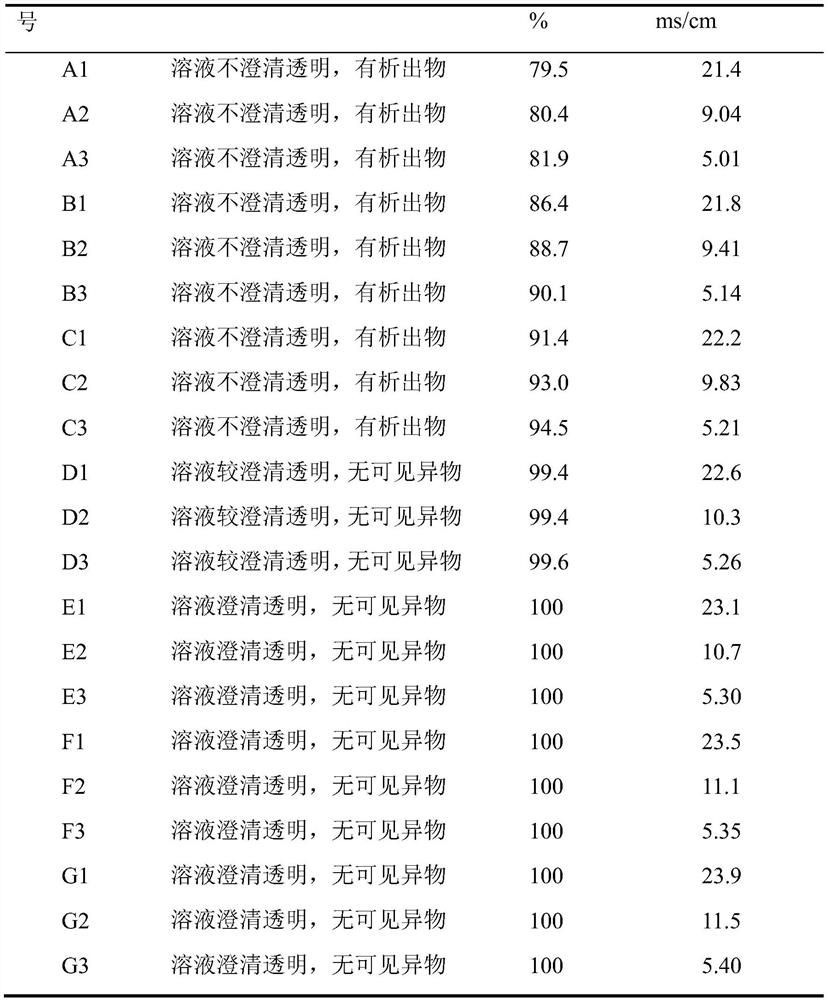
[0039] Take 4L of the solubilized dialysis internal solution in Example 1, use a membrane bag (2.5 square meters) with a cut-off of 10kD to perform ultrafiltration and phase change, first concentrate to 2L, add 2L of dialysis external solution 1 to mix and then concentrate to 2L, and then Use dialysis fluid 1 for dialysis phase change, keep the ultrafilter permeation rate and dialysis fluid addition rate at 2.5 L / h, and dialysis phase change for 4 hours. During 2h25min, 3h15min, and 4h of the dialysis phase change process, 10ml of dialysis fluid was taken respectively, and samples A1, A2, and A3 were marked.
Embodiment 3
[0040] Example 3 Using an ultrafilter to dialyze the recombinant human tissue-type plasmin kinase derivative inclusion body enhancement solution
[0041] Take 4L of the solubilized dialysis internal solution in Example 1, use a membrane bag (2.5 square meters) with a cut-off of 10kD to perform ultrafiltration and phase change, first concentrate to 2L, add 2L of dialysis external solution 2 to mix and then concentrate to 2L, and then Use dialysis fluid 2 for dialysis phase change, keep the ultrafilter permeation rate and dialysis fluid addition rate both at 2.5 L / h, and dialysis phase change for 4 hours. During 2h25min, 3h15min, and 4h of the dialysis phase change process, 10ml of the dialysis fluid was taken respectively, and samples B1, B2, and B3 were marked.
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com