Benzimidazole fluorescent probe for trifluralin detection and synthesis method thereof
A benzimidazole, fluorescent probe technology, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problem of few fluorescence detection methods, and achieve the effect of high selectivity and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Preparation of compound 1
[0019]
[0020] In a 100mL round bottom flask, add 3.12g of vanillin, 1.08g of o-phenylenediamine and 25mL of anhydrous methanol, heat to reflux for 6h, cool down to room temperature, the system precipitates a solid, filter, the filter cake is washed with anhydrous methanol, and dried. 1.88 g of white solid (namely compound 1) was obtained with a yield of 50%.
[0021] HRMS (ESI) C 22 h 20 N 2 o 4 calcd.for[M+H] + 377.1501; found 377.1482.
[0022] Preparation of compound 2
[0023]
[0024] In a 50 mL round bottom flask, add 0.37 g of compound (1), 0.69 g of N,N-diphenyl chloride formamide and 20 mL of pyridine, stir at 60°C for 12 h, and monitor the reaction with a TLC plate. After the reaction was completed, the mixture was washed successively with dilute HCl and saturated NaCl solution, and then extracted three times with dichloromethane. The organic layers were combined, and the organic phase was dried with anhydrous sodiu...
example 2
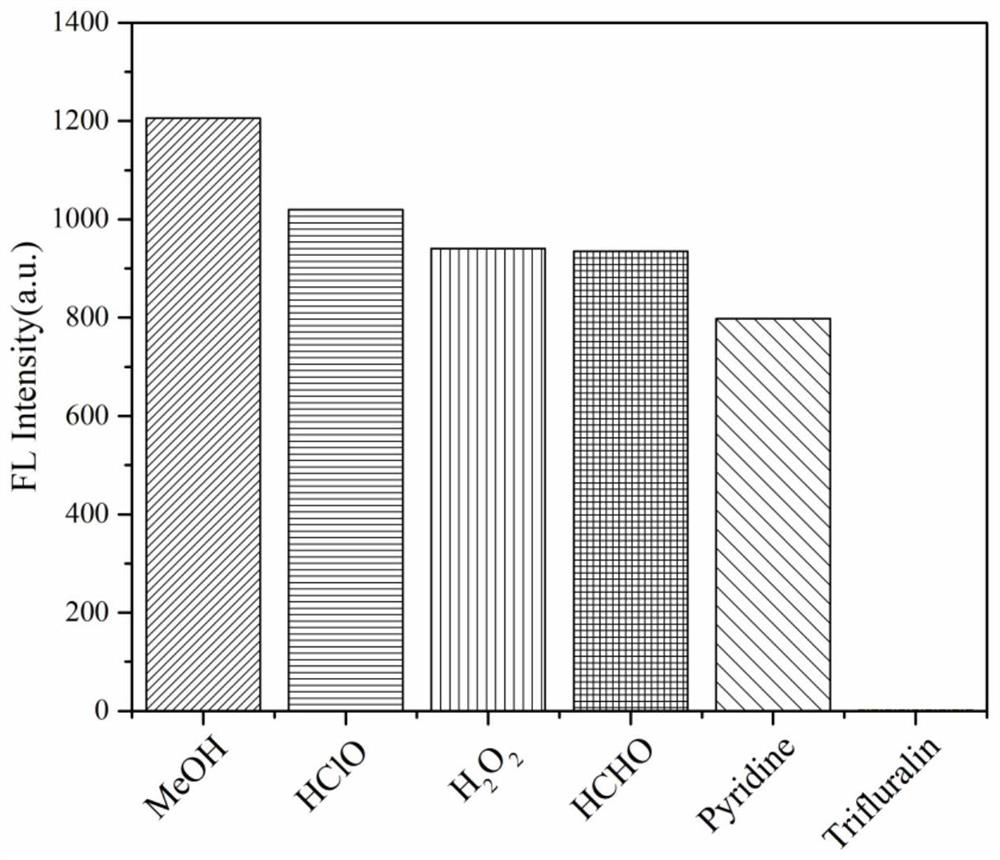
[0027] In compound 2 10 -5 10 equivalents of hypochlorous acid, hydrogen peroxide, formaldehyde, pyridine and trifluralin were respectively added to the methanol solution of M, and the changes in fluorescence emission intensity at 292 nm were measured. It was found that after adding 10 equivalents of different organic substances, compound 2 had good selectivity to trifluralin, and the fluorescence was quenched at 350 nm, such as figure 1 and figure 2 shown.
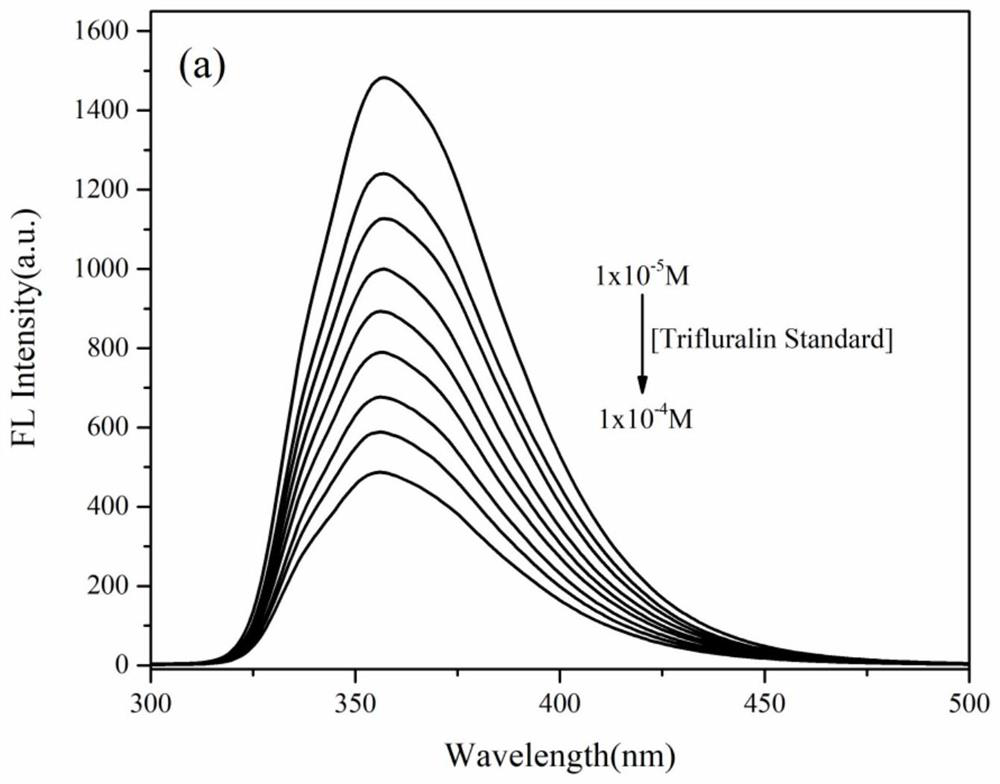
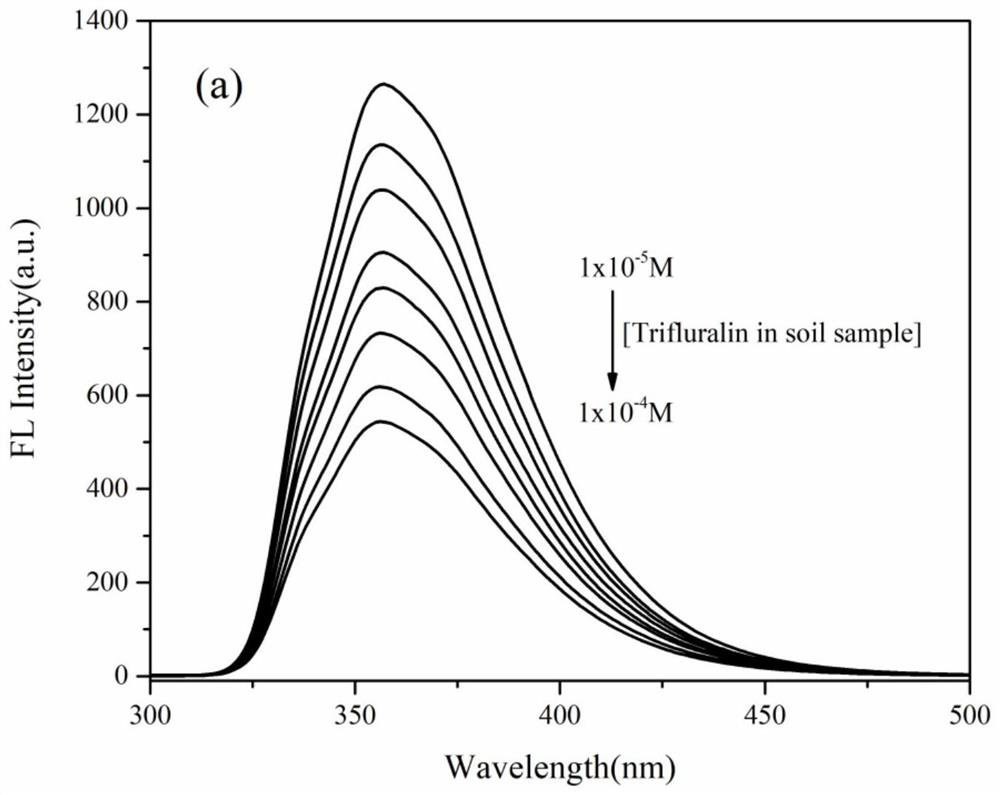
[0028] In compound 2 10 -5 The methanol solution of M was added different concentrations of trifluralin standard solution and the soil sample solution was obtained by leaching the trifluralin soil sample solution for fluorescence titration experiments. It was found by comparison that compound 2 was practical and feasible as a fluorescent probe for trifluralin detection. ,Such as figure 2 and image 3 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com