Molecular markers of colorectal cancer in blood and its detection kit and method
A molecular marker, colorectal cancer technology, applied in biochemical equipment and methods, microbial determination/inspection, DNA/RNA fragments, etc., can solve problems such as influence detection, poor specificity, and large influence of diet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] A method for detecting molecular markers of colorectal cancer in blood, comprising the steps of:
[0066] 1. Whole Blood Processing
[0067] 1.1 Use EDTAK2 anticoagulant vacuum blood collection tube (BD, Cat#367525) to collect 10mL whole blood, mix well,
[0068] Avoid hemolysis and process whole blood for plasmapheresis within 4-6 hours.
[0069] 1.2 Whole blood was centrifuged in a low-speed centrifuge at 4°C, 1600g, for 15 minutes, and the upper layer of plasma was carefully absorbed to avoid absorbing the buffy coat in the middle. The obtained plasma was centrifuged again in a high-speed centrifuge at 4°C, 16000g, for 10 minutes to obtain Sample plasma.
[0070] 2. Extraction of cfDNA from plasma
[0071] Specific method: The specific operation steps of plasma DNA extraction follow the MagMAX of Thermo Fisher Company TM Cell-Free DNA Isolation Kit instructions.
[0072] 3. Sulphite conversion of extracted cfDNA
[0073] The extracted DNA is subjected to bisul...
Embodiment 2
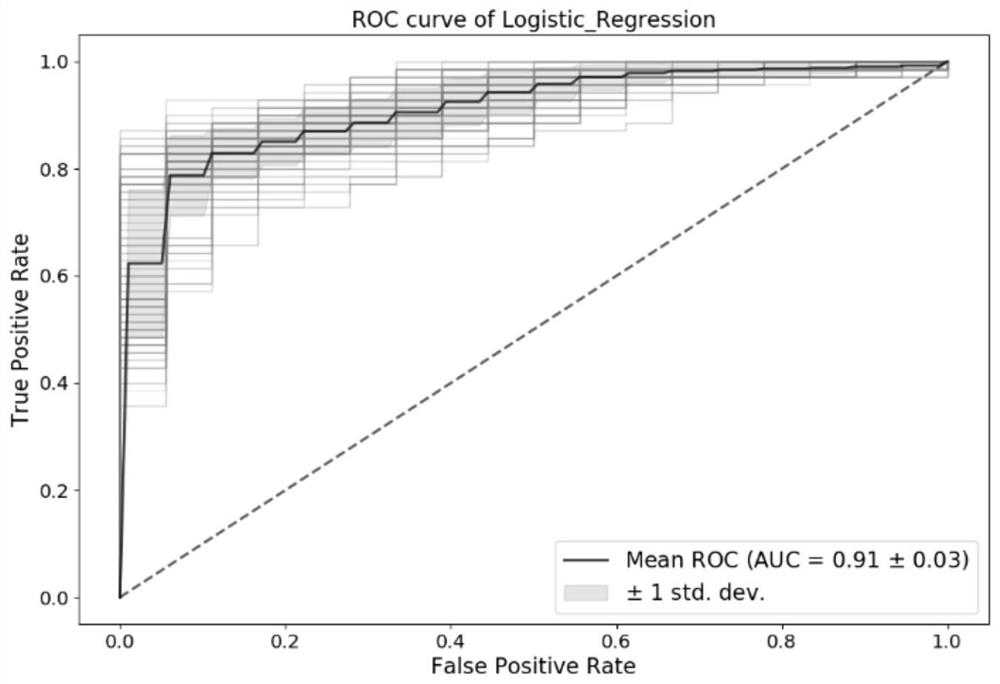
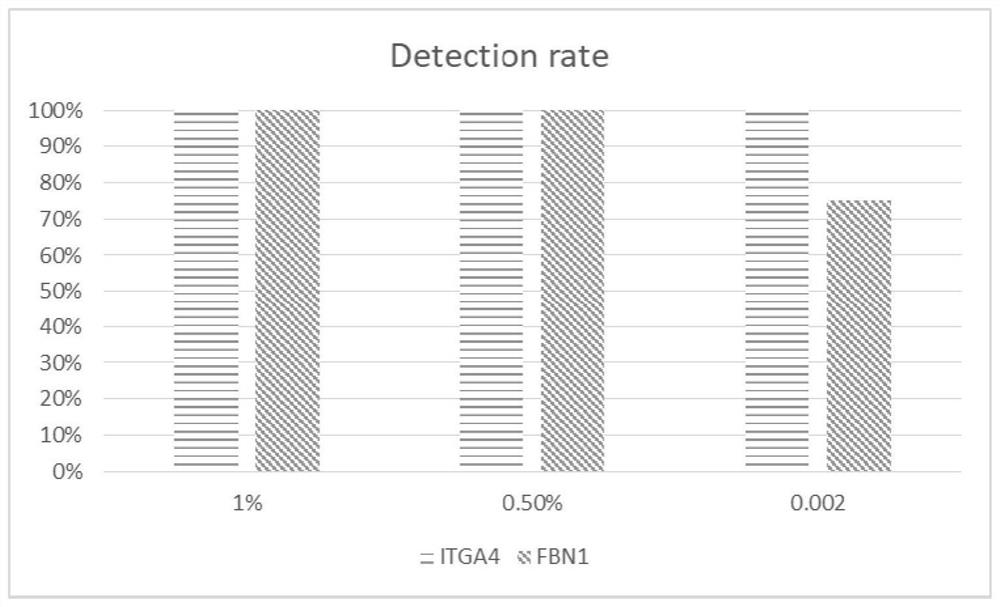
[0098] Using the experimental method of Example 1, the plasma samples of 46 normal people without colorectal cancer and 175 patients with colorectal cancer were tested. The specific detection kits, experimental methods and data judgment and processing were consistent with those in Example 1. Combinations of primers and probes are also preferably used in Example 1. 10 markers (TWIST1, VAV3-AS1, FBN1, C9orf50, SFMBT2, KCNQ5, FAM72C, ITGA4, KCNJ12, ZNF132) were selected for modeling and analysis using the logistic regression model, and 100 repetitions were performed according to the division of 6:4. At 90% specificity, the detection sensitivity of the test set was 78 ± 12% for Phase 1, 77 ± 10% for Phase 2, 82 ± 11% for Phase 3, and 88% for Phase 4 ±5%, for all colorectal cancer samples, the overall sensitivity of detection was 85±5%. The overall AUC was 0.91 ± 0.03. It shows that these markers are highly correlated with colorectal cancer, and through this method, colorectal ca...
Embodiment 3
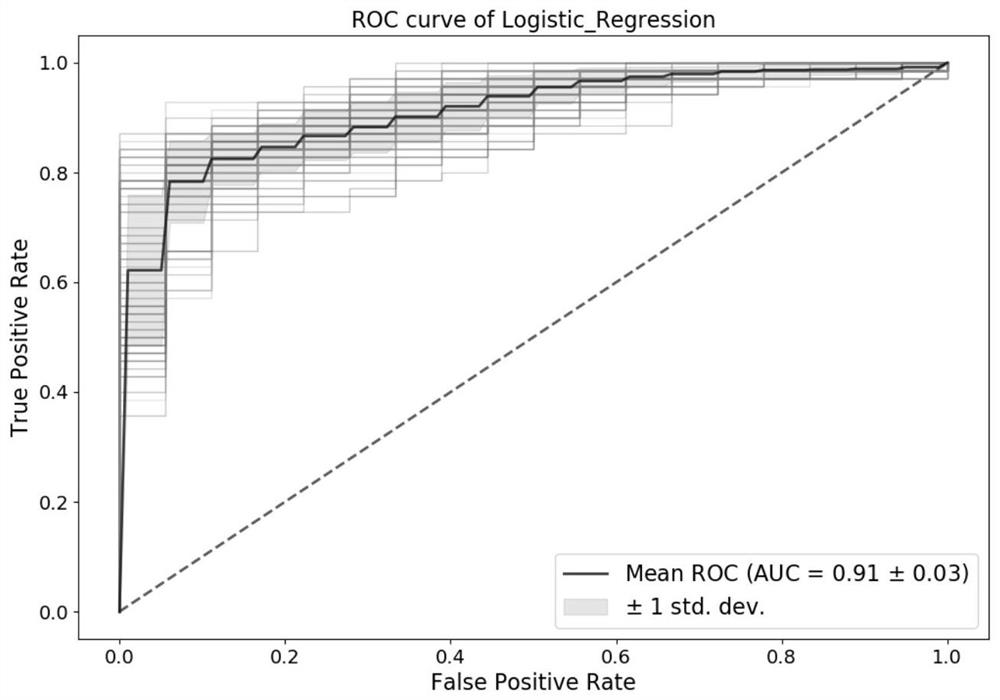
[0100] The detection kit and experimental method of Example 1 were used to detect the plasma samples of 46 normal people without colorectal cancer and 175 patients with colorectal cancer. The specific experiments and data judgment and processing were consistent with those in Example 1. The primers The combination with the probe is also preferably used in Example 1. Select 4 markers (TWIST1, C9orf50, KCNJ12, ZNF132) to use the logistic regression model for modeling analysis, according to the 6:4 segmentation, 100 repetitions, under 90% specificity, the detection of the first phase of the test set The sensitivity was 78 ± 12%, the detection sensitivity of phase 2 was 76 ± 10%, the detection sensitivity of phase 3 was 81 ± 11%, and the detection sensitivity of phase 4 was 88 ± 5%. For all colorectal cancer samples, the detected The overall sensitivity was 82±5%. The overall AUC was 0.91 ± 0.03. It shows that these markers are highly correlated with colorectal cancer, and throug...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com