Method for determining content of bis-(N-bis(dimethylamino)methylene)-iminium chloride catalyst
A technology of imide chloride salt and dimethylamine group, applied in the field of analytical chemistry, can solve the problems of not finding a method for catalyst quality control, unable to accurately grasp the real amount of catalyst, expensive catalyst and other problems, achieving fast determination and easy operation. , the effect of small sample size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] 1. There is a batch of self-synthesized bis-(N-bis(dimethylamino)methylene)-imide chloride salt catalyst samples. After standing for a period of time, the appearance of the sample absorbs moisture. Synthesizers need to know before using the catalyst The exact content is analyzed by proton nuclear magnetic spectroscopy, and the specific steps include:
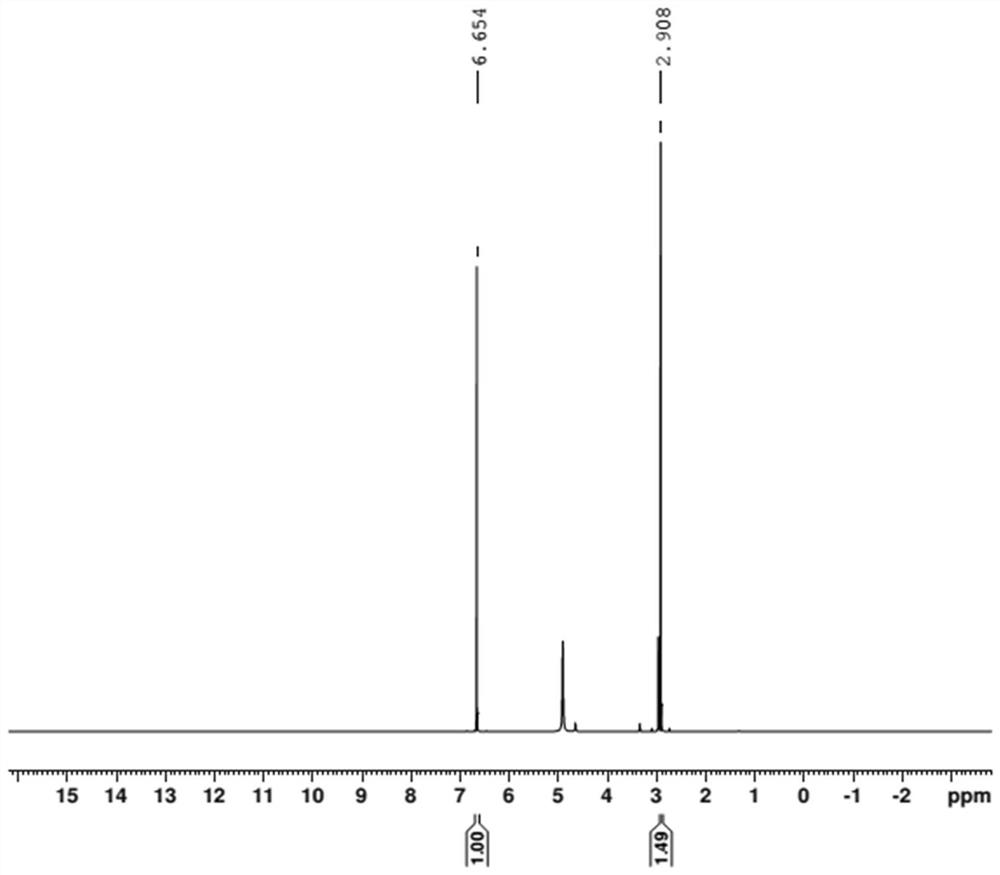
[0039] (1) Accurately weigh 29.22 mg of internal standard hydroquinone and 27.54 mg of bis-(N-bis(dimethylamino)methylene)-imide chloride salt into the sample bottle, add 0.6 mL of deuterated methanol After dissolving, transfer to NMR tube for H NMR test. The resonant frequency of the nuclear magnetic resonance spectrometer is 400MHZ, the pulse dump angle is 30 degrees, the test temperature is 30°C, the spectrum width is 10ppm, and the delay time is 15s.
[0040] (2) if figure 1 As shown, the methyl resonance peak at the chemical shift of δ=2.91ppm was selected as the quantitative peak of the catalyst sample. The catal...
Embodiment 2
[0042] 1. There is a batch of self-synthesized bis-(N-bis(dimethylamino)methylene)-imide chloride salt catalyst samples. After standing for a period of time, the appearance of the sample absorbs moisture. Synthesizers need to know before using the catalyst The exact content is analyzed by proton nuclear magnetic spectroscopy, and the specific steps include:
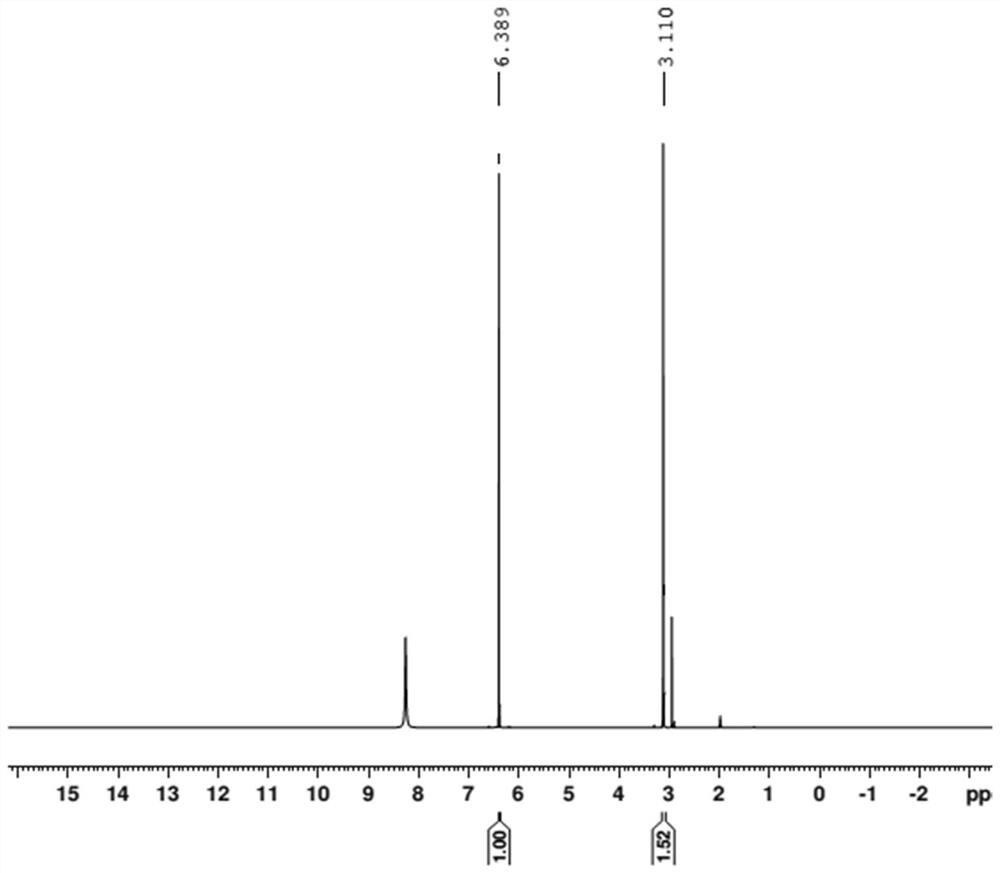
[0043] (1) Accurately weigh 41.50 mg of internal standard maleic acid and 17.63 mg of bis-(N-bis(dimethylamino)methylene)-imide chloride salt into a sample bottle, add 0.6 mL of deuterated acetonitrile to dissolve Afterwards, it was transferred to an NMR tube for the H NMR spectrum test. The resonant frequency of the nuclear magnetic resonance spectrometer is 400MHZ, the pulse dump angle is 30 degrees, the test temperature is 25°C, the spectrum width is 10ppm, and the delay time is 20s.
[0044] (2) if figure 2 As shown, the methyl resonance peak at the chemical shift of δ=3.11ppm was selected as the quantitative peak of...
Embodiment 3
[0046] 1. There is a batch of self-synthesized bis-(N-bis(dimethylamino)methylene)-imide chloride salt catalyst samples. After standing for a period of time, the appearance of the sample absorbs moisture. Synthesizers need to know before using the catalyst The exact content is analyzed by proton nuclear magnetic spectroscopy, and the specific steps include:
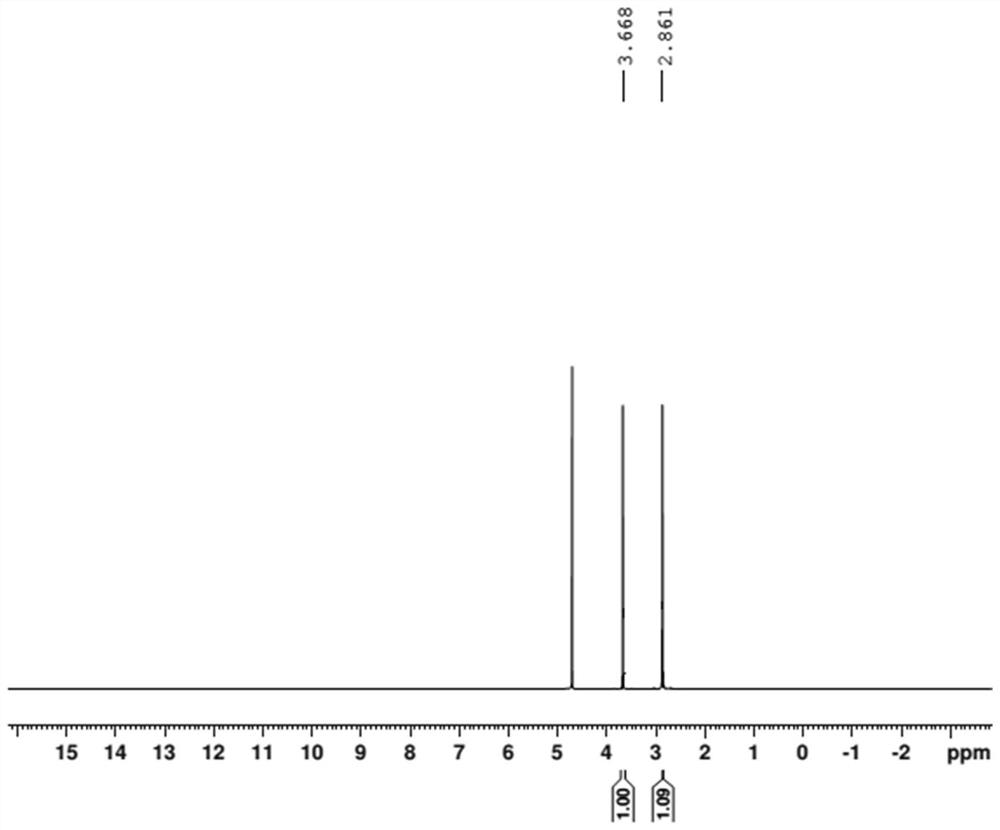
[0047] (1) Accurately weigh 6.87 mg of internal standard 1,4-dioxane and 13.25 mg of bis-(N-bis(dimethylamino)methylene)-imide chloride salt into the sample bottle, add 0.6 mL of deuterium water was dissolved and transferred to an NMR tube for proton NMR testing. The resonant frequency of the nuclear magnetic resonance spectrometer is 400MHZ, the pulse dump angle is 30 degrees, the test temperature is 23°C, the spectrum width is 10ppm, and the delay time is 25s.
[0048] (2) if image 3 As shown, the methyl resonance peak at the chemical shift of δ=2.86ppm was selected as the quantitative peak of the catalyst sample. T...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Resonant frequency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com