System and method for preparing 2, 3, 3, 3-tetrafluoropropene
A technology of tetrafluoropropylene and tetrachloroethylene, which is applied in the field of chemical preparation, can solve the problems of excessive waste water, corrosion, and high reaction temperature, and achieve high raw material conversion rate, high mass and heat transfer efficiency, and safe and controllable reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] Preparation of fluorination catalyst
[0034] 40g (0.1mol) Cr(NO 3 ) 3 9H 2 O, 24g (0.067mol) Al(NO 3 ) 3 9H 2 O is configured into mixed solution 1 with deionized water, and 12.8g (0.05mol) of Mg(NO 3 ) 2 ·6H 2 O, 19.7g (0.05mol) Zn(NO 3 ) 2 ·6H 2 O, 8.4g (0.05mol) FeCl 3 Make a mixed solution with deionized water, stir, add hydrochloric acid dropwise until the solid dissolves, and configure mixed solution 2. Under stirring, the mixed solution 2 was mixed with the mixed solution 1, 10% ammonia solution was added dropwise until the pH was about 10, and the reaction temperature was kept at 50° C. for 1 h. Filter and wash the filter cake with deionized water until neutral; take the filter cake and dry it overnight at 120°C. The obtained dried solid was placed in a muffle furnace and calcined at 600° C. for 2 hours to obtain a catalyst precursor.
[0035]Extrude the obtained catalyst precursor into a cylindrical shape with an extruder, fill it into a stainles...
Embodiment 1
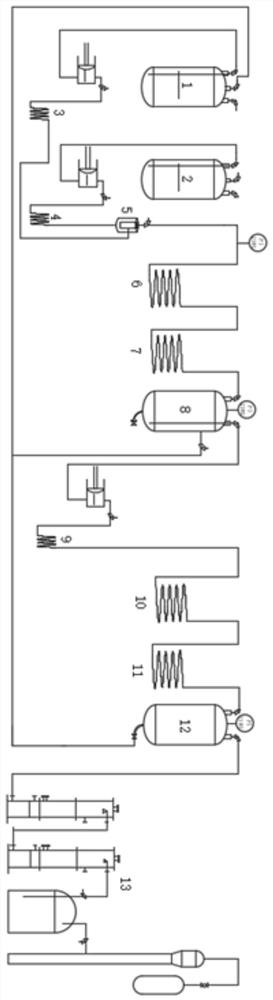
[0040] Such as figure 1 As shown, a system for preparing 2,3,3,3-tetrafluoropropene includes a hydrogen fluoride storage tank 1, a 1,1,2,3-tetrachloroethylene storage tank 2, a first heater 3, a second heating Device 4, mixer 5, fluorination reactor 6, first cooling device 7, first separation tank 8, third heater 9, elimination tube reactor 10, second cooling device 11, second separation tank 12, Purification device 13, wherein the hydrogen fluoride storage tank 1 and the 1,1,2,3-tetrachloroethylene storage tank 2 pass through the first heater 3 and the second heater 4 and the mixer 5 respectively connected, the mixer 5 sequentially passes through the fluorination reactor 6, the first cooling device 7, the first separation tank 8, the third heater 9, the elimination tube reactor 10, The second cooling device 11 and the second separation tank 12 are connected to the purification device 13 .
Embodiment 2
[0042] 1,1,2,3-tetrachloroethylene flows through the first heater 3 at a flow rate of 0.1m / s, and heats up to 300°C; hydrogen fluoride flows through the second heater 4 at a flow rate of 0.6m / s, and heats up to 300°C ; Then 1,1,2,3-tetrachloroethylene through the first heater 3 and hydrogen fluoride through the second heater 4 are mixed into the tubular reactor 6 filled with a fluorination catalyst, reacted at 350° C., and the residence time is 30s , enter the first cooling device 7 to cool to normal temperature, cool to normal temperature, enter the first separation tank 8, exhaust, and maintain a pressure of 0.5Mpa;
[0043] The lower layer liquid 1,1,1,2,3-pentafluoropropane (HFC-245eb) in the first separation tank 8 enters the third heater 9 at a flow rate of 0.1m / s, and is heated to 300°C; In the tubular reactor 10 of the catalyst, react at 400°C, the residence time is 6s, enter the second cooling device 11 to cool to normal temperature, enter the second separation tank 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com