Method for preparing triuranium octaoxide from uranium hexafluoride
A technology of triuranium eight oxide and uranium hexafluoride, which is applied in the field of nuclear fuel cycle, can solve the problems of increasing fluorine content, large amount of waste water, and long wet process, and achieves low fluorine content, reduction of ammonia nitrogen waste water, and convenient The effect of long-term stable storage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Uranyl solution: uranium 138.5g / L, fluorine 22.7g / L;
[0033] Step one, UO 2 f 2 Preparation of hydrolyzate;
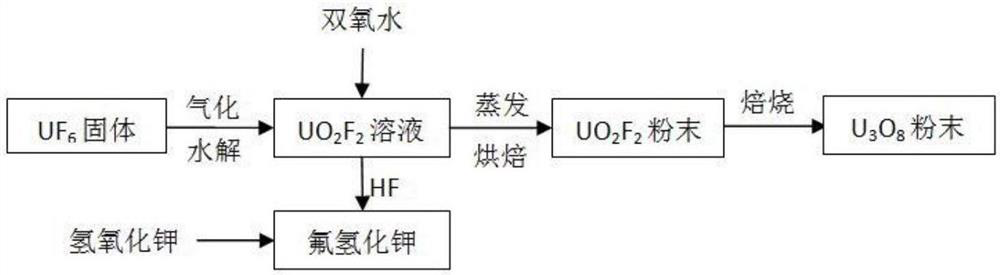
[0034] The solid UF6 is heated and vaporized and slowly passed into the water washing equipment for hydrolysis reaction to prepare UO 2 f 2 solution.
[0035] Step two, UO 2 f 2 powder preparation;
[0036] Add a certain amount of hydrogen peroxide solution (mass concentration 30%) to the uranyl solution, react and then put the solution into the evaporator, slowly heat and concentrate to form UO 2 f 2 crystallized and subsequently baked into dry UO 2 f 2 Powder, the HF gas in the evaporation process is passed into the potassium hydroxide solution to prepare the by-product potassium bifluoride. The amount of hydrogen peroxide added to the reaction control is 10%, and the baking temperature is 150°C.
[0037] Step 3, roasting and preparing U 3 o 8 ;
[0038] Dry UO 2 f 2 The powder is placed in a roasting furnace and oxidized to U in an aerobic h...
Embodiment 2
[0041] Uranyl solution: uranium 138.5g / L, fluorine 22.7g / L
[0042] Step one, UO 2 f 2 Preparation of hydrolyzate;
[0043] The solid UF6 is heated and vaporized and slowly passed into the water washing equipment for hydrolysis reaction to prepare UO 2 f 2 solution.
[0044] Step two, UO 2 f 2 powder preparation;
[0045] Add a certain amount of hydrogen peroxide solution (mass concentration 30%) to the uranyl solution, react and then put the solution into the evaporator, slowly heat and concentrate to form UO 2 f 2 crystallized and subsequently baked into dry UO 2 f 2 Powder, the HF gas in the evaporation process is passed into the potassium hydroxide solution to prepare the by-product potassium bifluoride. The amount of hydrogen peroxide added for reaction control is 10%, and the baking temperature is 150°C;
[0046] Step 3, roasting and preparing U 3 o 8 ;
[0047] Dry UO 2 f 2 The powder is placed in a roasting furnace and oxidized to U in an aerobic high-...
Embodiment 3
[0050] Uranyl solution: uranium 138.5g / L, fluorine 22.7g / L
[0051] Step one, UO 2 f 2 Preparation of hydrolyzate;
[0052] The solid UF6 is heated and vaporized and slowly passed into the water washing equipment for hydrolysis reaction to prepare UO 2 f 2 solution.
[0053] Step two, UO 2 f 2 powder preparation;
[0054] Add a certain amount of hydrogen peroxide solution (mass concentration 30%) to the uranyl solution, react and then put the solution into the evaporator, slowly heat and concentrate to form UO 2 f 2 crystallized and subsequently baked into dry UO 2 f 2 Powder, the HF gas in the evaporation process is passed into the potassium hydroxide solution to prepare the by-product potassium bifluoride. The amount of hydrogen peroxide added for reaction control is 10%, and the baking temperature is 150°C;
[0055] Step 3, roasting and preparing U 3 o 8 ;
[0056] Dry UO 2 f 2 The powder is placed in a roasting furnace and oxidized to U in an aerobic high-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com