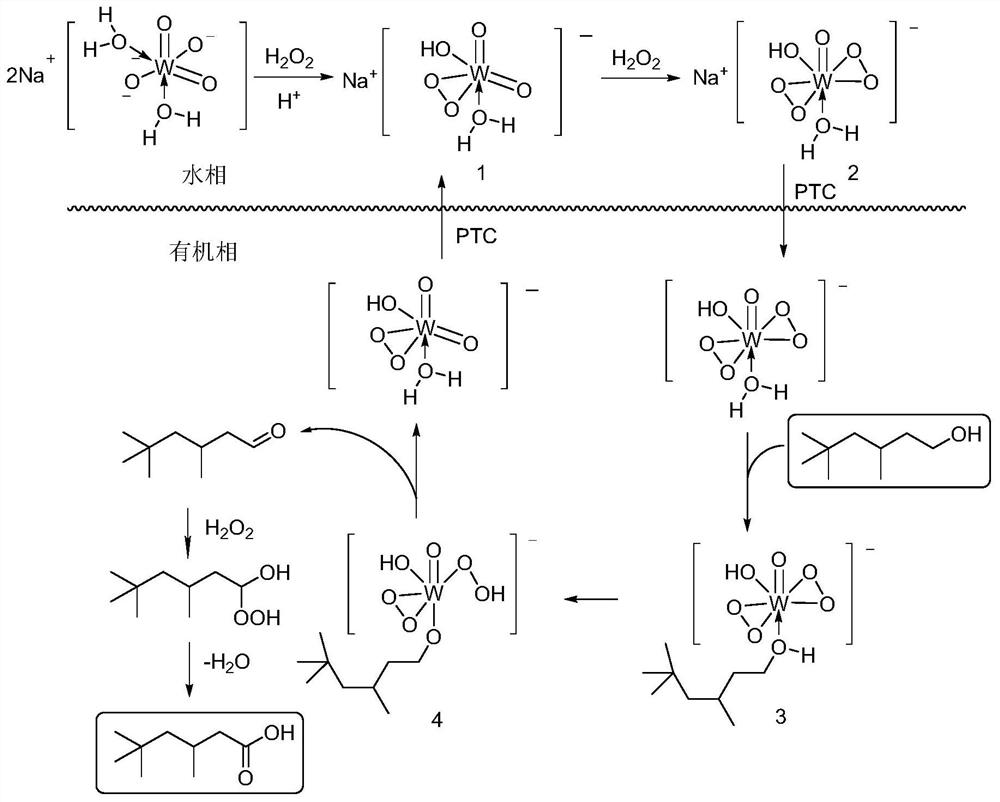
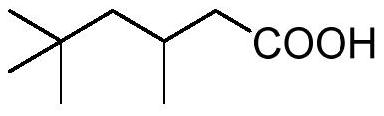
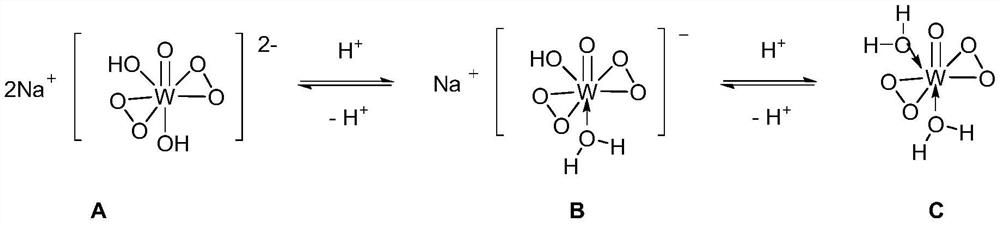
Method for preparing isononanoic acid from isononyl alcohol through green oxidation
A technology of isononyl alcohol and isononanoic acid, applied in the field of biomedicine, can solve the problems of high pressure, low activity and selectivity of phosphine compound ligands, complicated operation process, etc., achieves the improvement of reaction product yield, avoids excessive oxidation and Effects of explosion risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] In a 100mL two-necked round bottom flask equipped with a reflux condenser and a thermometer, add isononyl alcohol (14.42g, 100mmol), 30% H 2 o 2 (8.50g, 250mmol), Na 2 WO 4 2H 2 O (0.66g, 2mmol) and methyl trioctyl ammonium bisulfate (0.93g, 2mmol) were mixed uniformly, measured with precision pH test paper and adjusted to a pH value of about 1. Stir rapidly and heat to 90°C, maintain the reaction temperature of 90°C for 4 hours, add water and let it stand after cooling to room temperature, separate the supernatant and carry out vacuum distillation to obtain 14.40 g of isononanoic acid product with a yield of 91%. 1 H NMR (400MHz, CDCl 3 )δ0.87(s, 9H), 1.02(d, J=6.4Hz, 3H), 1.12-1.34(m, 2H), 2.06-2.40(m, 3H); 13 C NMR (100MHz, CDCl 3)δ22.6, 26.8, 29.9, 31.0, 43.7, 50.5, 179.4, the NMR spectrum is completely consistent with the standard spectrum.
Embodiment 2
[0040] In a 100mL two-necked round bottom flask equipped with a reflux condenser and a thermometer, add isononyl alcohol (14.42g, 100mmol), 30% H 2 o 2 (6.80g, 200mmol), Na 2 WO 4 2H 2 O (0.66g, 2mmol) and methyl trioctyl ammonium bisulfate (0.93g, 2mmol) were mixed uniformly, measured with precision pH test paper and adjusted to a pH value of about 1. Stir rapidly and heat to 90°C, maintain the reaction temperature of 90°C for 4 hours, add water and let it stand after cooling to room temperature, separate the supernatant and carry out vacuum distillation to obtain 11.07g of isononanoic acid product with a yield of 70%. 1 H NMR (400MHz, CDCl 3 )δ0.87(s, 9H), 1.02(d, J=6.4Hz, 3H), 1.12-1.34(m, 2H), 2.06-2.40(m, 3H); 13 C NMR (100MHz, CDCl 3 )δ22.6, 26.8, 29.9, 31.0, 43.7, 50.5, 179.4, the NMR spectrum is completely consistent with the standard spectrum.
Embodiment 3
[0042] In a 100mL two-necked round bottom flask equipped with a reflux condenser and a thermometer, add isononyl alcohol (14.42g, 100mmol), 30% H 2 o 2 (10.20g, 300mmol), Na 2 WO 4 2H 2 O (0.66g, 2mmol) and methyl trioctyl ammonium bisulfate (0.93g, 2mmol) were mixed uniformly, measured with precision pH test paper and adjusted to a pH value of about 1. Stir rapidly and heat to 90°C, maintain the reaction temperature of 90°C for 4h, add water after cooling to room temperature, separate the supernatant and carry out vacuum distillation to obtain 13.92g of isononanoic acid product with a yield of 88%. 1 H NMR (400MHz, CDCl 3 )δ0.87(s, 9H), 1.02(d, J=6.4Hz, 3H), 1.12-1.34(m, 2H), 2.06-2.40(m, 3H); 13 C NMR (100MHz, CDCl 3 )δ22.6, 26.8, 29.9, 31.0, 43.7, 50.5, 179.4, the NMR spectrum is completely consistent with the standard spectrum.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com