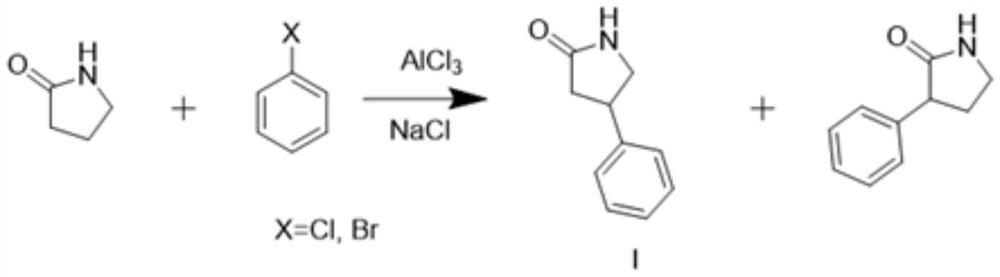
Synthesis method of 4-phenyl-2-pyrrolidone
A technology of pyrrolidone and a synthesis method, applied in the direction of organic chemistry and the like, can solve the problems of complex reaction conditions and low yield, and achieve the effects of simple reaction conditions, high yield and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
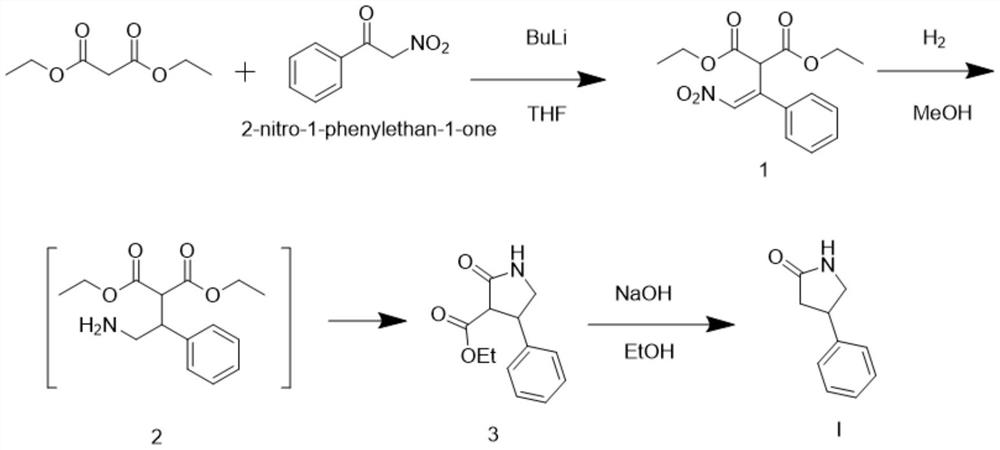
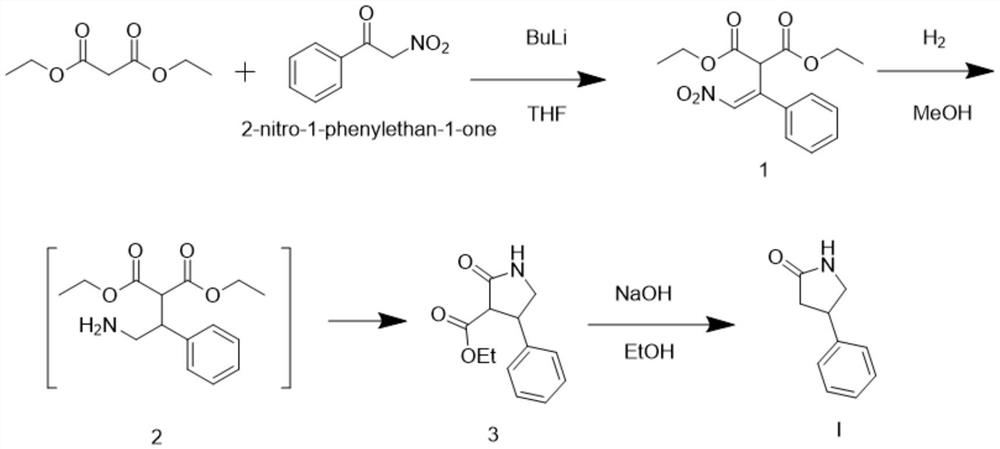
[0020] Embodiment 1 is the preparation embodiment of intermediate 1
[0021] Example 1
[0022] Add 16 grams of diethyl malonate, 300 milliliters of tetrahydrofuran and 16.5 grams of 2-nitro-1-phenethyl ketone into a 2000 milliliter single-necked flask. / L butyllithium 420 ml, reacted for 4 hours, after the reaction was completed, 200 ml of water was added dropwise, concentrated to remove the solvent, extracted with ethyl acetate, dried, and concentrated to obtain 30 g of intermediate 1. The yield is 92%.
Embodiment 2
[0025] Add 5 grams of intermediate 1, 20 milliliters of methanol and 0.1 grams of 10% palladium carbon into a 100 milliliter single-necked flask. Under the condition of stirring at 20-30 degrees, feed hydrogen gas and react for 2-3 hours. After the reaction is completed, filter the palladium carbon , the solution directly proceeds to the next reaction.
Embodiment 3
[0027] Add 5 grams of intermediate 1, 20 milliliters of ethanol and 0.1 grams of 10% palladium carbon into a 100 milliliter single-necked flask. Under the condition of stirring at 20-30 degrees, pass in hydrogen gas and react for 2-3 hours. After the reaction is completed, filter the palladium carbon , the solution directly proceeds to the next reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com